Abstract
Application of retrograde dissection method for isolation of bone marrow cells from rat femurs and tibiae
Author(s): C.M. Li, B.M. Fu, L.C. Zhang, B. Tang, L. Zhu, Y. Zhao and J. ZhangCurrently, there is no practical and efficient method for the isolation of bone marrow cells (BMCs) from rat femurs and tibiae. Here, we attempted to develop a rapid, simple, effective, and non-contaminating method for the isolation of BMCs from rat femurs and tibiae. Rat femurs and tibiae were dissected from the ankle to the hip joint; subsequently, a three-step “locate-slide-twist” procedure was performed using scissors and forceps to remove the femurs and tibiae completely, from the surrounding musculature. The bones were flushed with phosphate-buffered saline to harvest BMCs. The femurs and tibiae were dissected in 1.8 ± 0.6 min, and the BMC suspension preparation time was 13.1 ± 2.3 min. The bone marrow cavities did not incur any fractures or injuries during the isolation. Culture of harvested BMCs for 72 h led to a significant increase in cell number from 4.4 ± 0.3 x 106 to 6.9 ± 0.7 x 106 (P vs 96.2 ± 1.1%; P > 0.05). Microscopic examination of the isolated BMCs after the 72-h incubation period revealed the no-microbial or muscle cell contamination. Furthermore, flow cytometry revealed that cultured BMCs (72-h culture) grew well. Here, we have reported a rapid, simple, effective, and non-contaminating method for the isolation of BMCs from rat femurs and tibiae by using retrograde dissection. This method can be used to harvest a large number of viable BMCs without the risk of contamination from muscle and connective tissues.
Impact Factor an Index

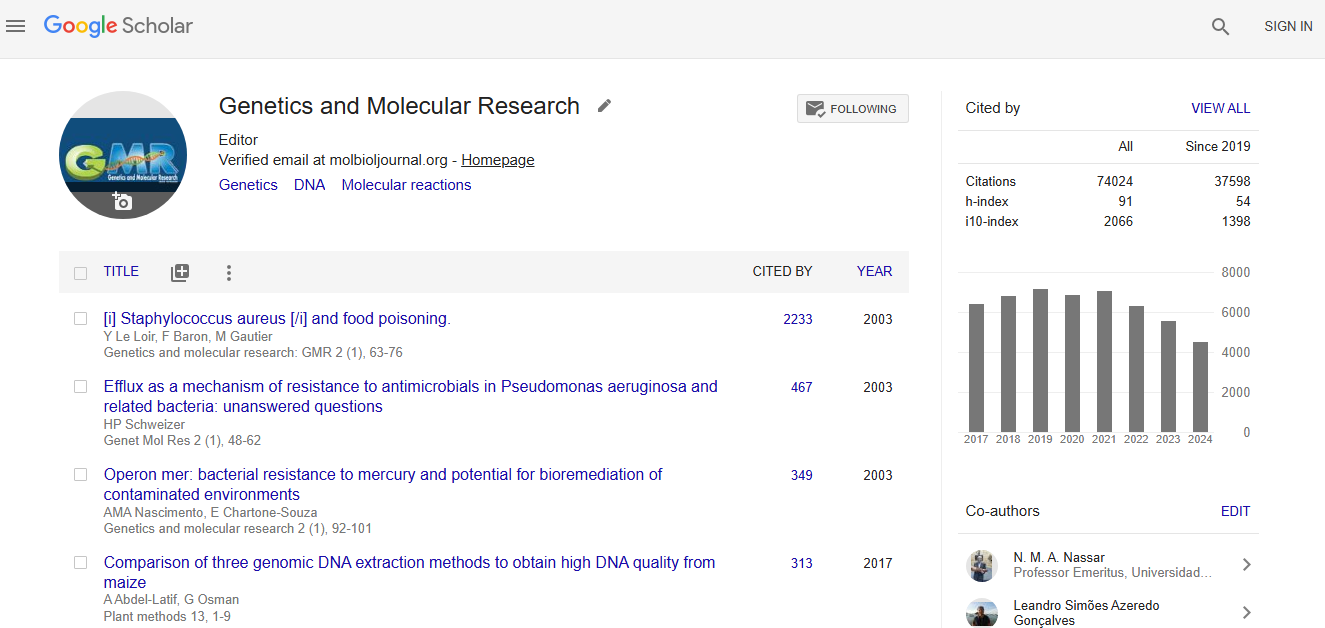
Google scholar citation report
Citations : 74024
Genetics and Molecular Research received 74024 citations as per google scholar report