Abstract
Expression and diagnostic value of proteins in Mycobacterium tuberculosis
Author(s): Z.Y. Zhu, Y. Liu, H.B. Wang, J.Z. Xiao, Y.F. Qiu, L. Yan, D. Chen, A.G. Liu and X. YangWe constructed a prokaryotic expression vector expressing the Mycobacterium tuberculosis protein TB16.3, as well as 3 other proteins, including TB15.3, CFP-10, and Rv2626C, which were purified and analyzed for their effectiveness as detection antibodies. The TB16.3 genes of M. tuberculosis H37Rv genomic DNA were amplified by polymerase chain reaction, inserted into the expression vector pET-30a, and expressed in Escherichia coli. An enzyme-linked immunosorbent assay was used to detect the 4 M. tuberculosis antibodies. Engineered E. coli bacteria expressing TB16.3 and the 3 other proteins were constructed and found mainly to be soluble. For recombinant TB16.3 proteins, serum samples of 118 tuberculosis (TB) patients and 96 healthy controls were analyzed. Sensitivity, specificity, and adjusted concordance rate for the TB16.3 antibody were 72.9, 86.5, and 79.6%, respectively. The positive rate of Rv2626C antibody in TB patients (44.1%) was significantly lower than that in normal controls (75.0%, χ2 = 20.8, P < 0.01). TB15.3 and TB16.3 were used for simultaneous detection and showed sensitivity, specificity, and repeatability rates of 69.4, 96.9, and 83.7%. The antibody positive rate and specificity for patients with lung disease was 9.6 and 90.4%, respectively. TB15.3 and TB16.3 were mixed and detected simultaneously. Combined with the results for TB15.3, the sensitivity, specificity, and concordance rates were 82.2, 95.9, and 88.9%, respectively. The concordance rate was the highest value observed. Target genes were cloned into a host strain and expressed successfully. The TB16.3 recombinant protein may be used as a new serological antigen for tuberculosis diagnosis.
Impact Factor an Index

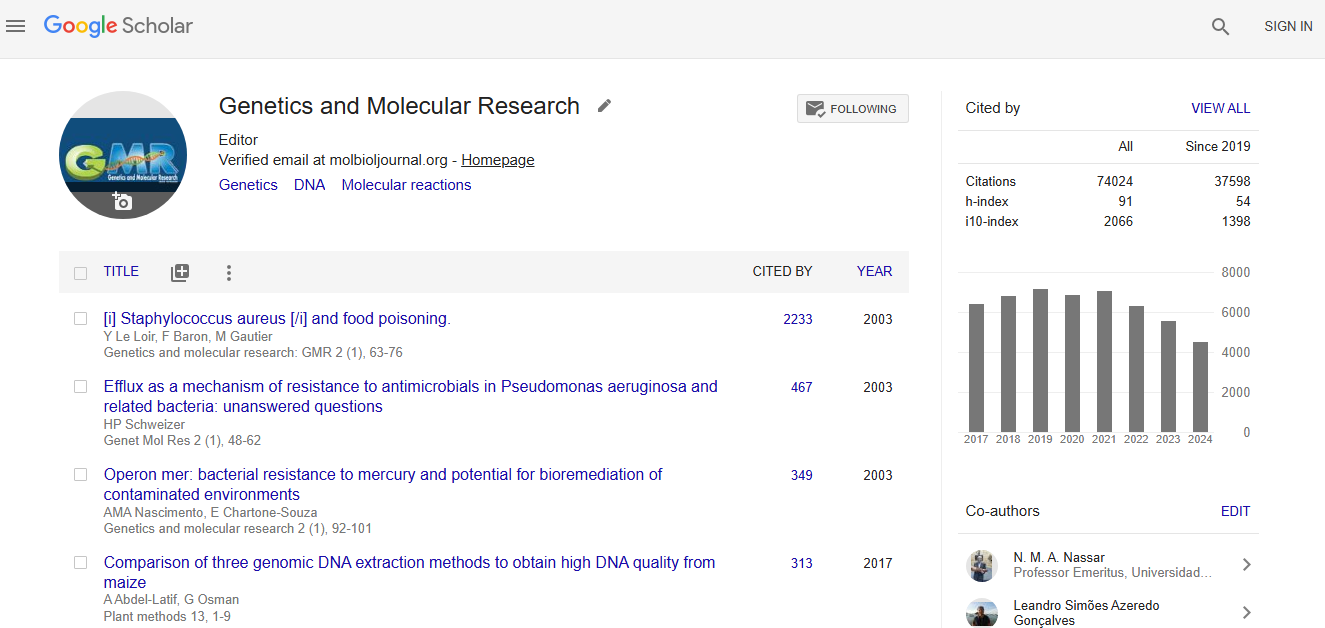
Google scholar citation report
Citations : 56184
Genetics and Molecular Research received 56184 citations as per google scholar report