Abstract
Expression and purification of GST-FHL2 fusion protein
Author(s): H. Yu, Q. Ma, J. Lin, Y.F. Sun and F. ZhengEscherichia coli is the most widely used host for the production of recombinant proteins. However, most eukaryotic proteins are typically obtained as insoluble, misfolded inclusion bodies that need solubilization and refolding. The interactions between human FHL2 protein and many types of proteins, including structural proteins, kinases, and several classes of transcription factor, have been found to have important roles in a variety of fundamental processes, including arrhythmia, hypertrophy, atherosclerosis, and angiogenesis. To achieve high-level expression of soluble recombinant human FHL2 protein in E. coli, we have constructed a recombinant expression plasmid, pGEX-4T-1-FHL2, in which we merged FHL2 cDNA with the glutathione S-transferase (GST) coding sequence downstream of the tac inducible promoter. Using this plasmid, we have achieved high expression of soluble FHL2 as a GST fusion protein in E. coli BL21. We have used the engineered plasmid (pGEX-4T-1-FHL2) and the modified E. coli strain to overcome the problem of removing the GST moiety while expressing soluble FHL2. Our results show that: 1) the recombinant plasmid was successfully constructed.
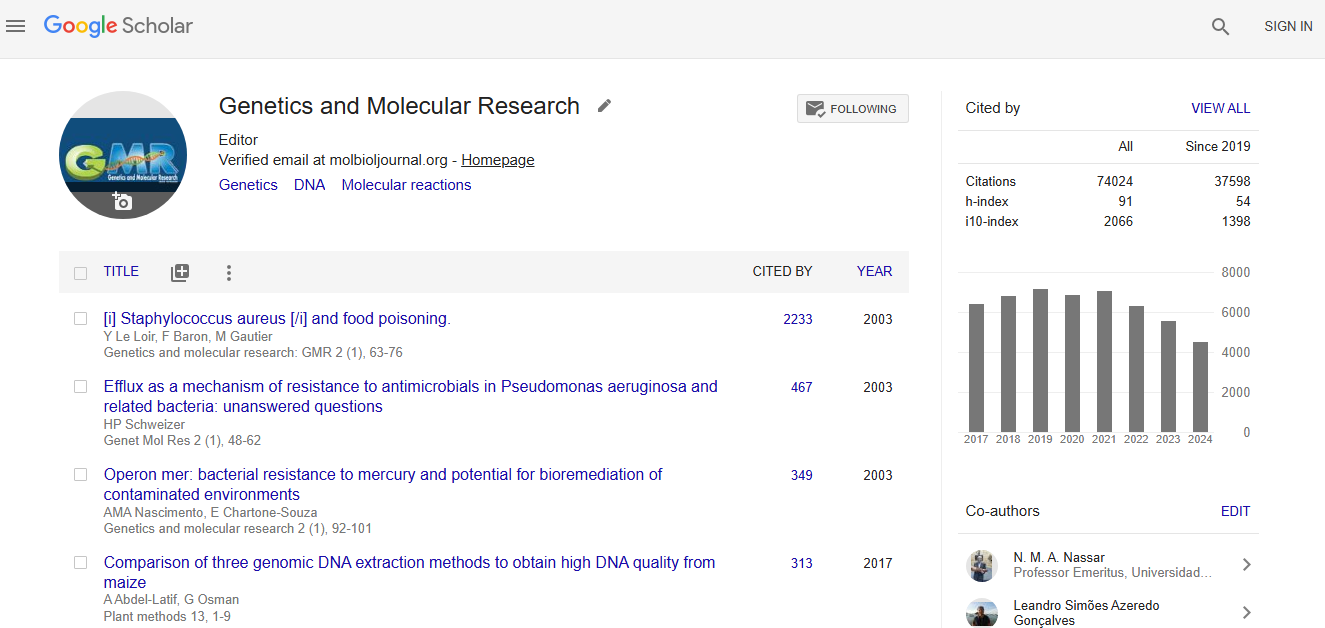
Impact Factor an Index

Google scholar citation report
Citations : 74024
Genetics and Molecular Research received 74024 citations as per google scholar report