Abstract
Molecular cloning and characterization, and prokaryotic expression of the GnRH1 gene obtained from Jinghai yellow chicken
Author(s): T. Zhang, G.X. Zhang, K.P. Han, Y. Tang, J.Y. Wang, Q.C. Fan, X.S. Chen, Y. Wei and Y.J. WangThe gonadotropin-releasing hormone (GnRH) plays an important role in the control of reproductive functions. Recent studies have reported the occurrence of GnRH molecular variants in numerous species. In this study, the GnRH1 gene from Jinghai yellow chicken was cloned by reverse transcriptase-polymerase chain reaction and transformed into BL21 (DE3) competent cells. The GnRH1 gene and amino acid sequences were subjected to bioinformatic analyses. The GnRH1 gene nucleotide sequence was discovered to be 352 bp long, containing a coding, promoter, and section of the 3'-regions. The GnRH1 gene shared 93, 81, 54, 58, 61, 76, 76, 59, 76, and 66% sequence identity with Meleagris gallopavo, Columba livia, Homo sapiens, Bos taurus, swines, Capra hircus, Ovis aries, Pantholops hodgsonii, Equus caballus, and Rattus norvegicus, respectively. The GnRH1 gene showed conserved domains. The GnRH1 protein was a secreted protein comprising 92 amino acids, with a molecular weight of 10205.6 Da and a theoretical pI of 5.67. Most of the amino acid residues were observed to be hydrophilic, indicating water solubility. The predicted secondary structures of proteins included α-helices (h; 23.08%), β-extensions (e; 10.92%), and random coils (c; 66.0%). The successful construction of prokaryotic expression vector pET32a-GnRH1 was confirmed by restriction and sequence analysis. SDS-PAGE analysis showed the successful expression of recombinant plasmid in Escherichia coli BL21 (molecular weight = 25-28 kDa). Larger quantities of protein were expressed in supernatant, indicating greater expression in soluble form. Western blot analysis confirmed the expression of the target protein.
Impact Factor an Index

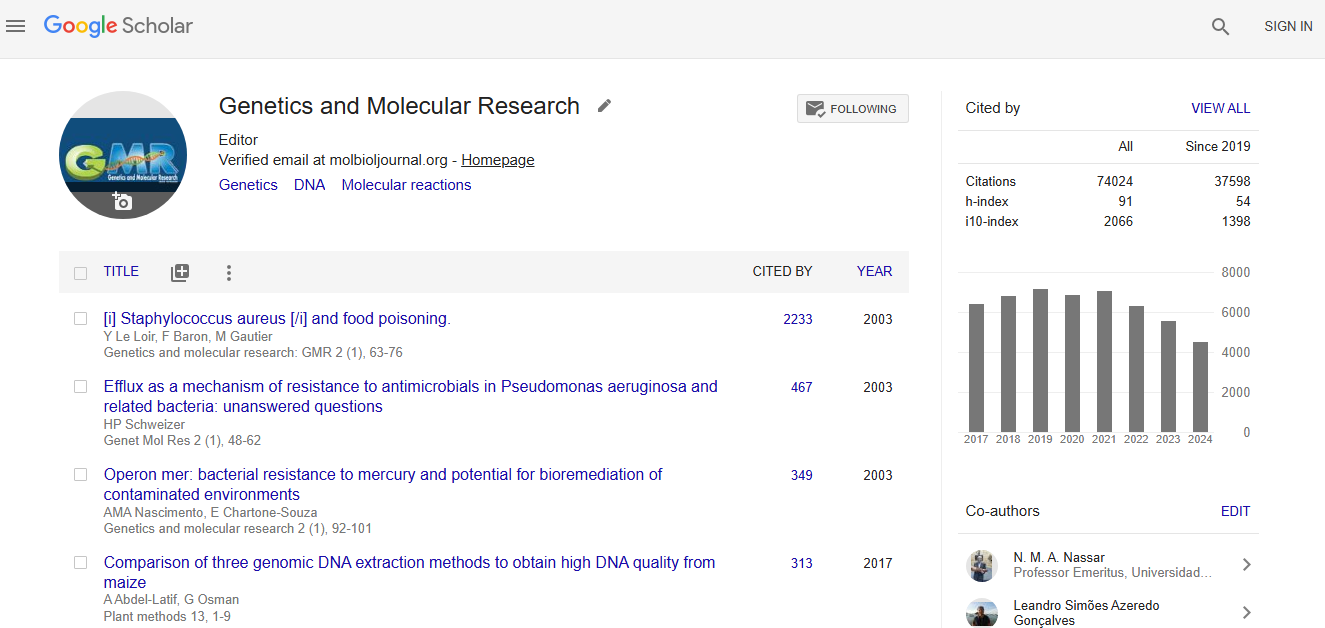
Google scholar citation report
Citations : 74024
Genetics and Molecular Research received 74024 citations as per google scholar report