Expression of HCN and sodium channels in murine model of Type I diabetes
Received: April 12, 2019
Accepted: April 13, 2019
Published: May 05, 2019
Genet.Mol.Res. 18(2):
Keywords
Type 1 Diabetes; Voltage gated sodium channel; Hyperpolarization activated cyclic nucleotide gated channel; Gene expression; Ion flux.
Introduction
Pancreatic apoptosis is an important process in Type 1 Diabetes, in which mostly immune cells and pancreatic islets in some instances, secrete enhanced amount of inflammatory cytokines like IL-1β, TNF-α and IFN-γ against the pancreatic islets (Wang et al., 2010). Although many factors like genetic, environmental factors have been identified as major contributors for this disease, the causative agents and the underlying mechanism are still largely unknown (Pirot et al., 2008).
Pancreatic cell death is a cyclic and complex event, initiated in response to binding of these cytokines to the specialized receptor, present on pancreatic cell. This activates many downstream signaling mechanisms that account for increased ER stress, NOS production and ultimately results in increased intracellular calcium concentration. This increased cytosolic calcium, in turn activates apoptotic inducing calcium dependent enzyme like caspases and calcineurin (Ramadan et al., 2011; Eizirik et al., 2001). This activated caspases induce the generation of cascade of apoptotic signaling events such as cleavage of BID protein to induce efficient cell death and the calcineurin modulate the phosphorylation of pro-apoptotic molecule Bad (Wang et al., 1999; McIlwain et al., 2013). For studying the underlying mechanism of elevated calcium concentration, we have taken the advantage of gene expression analysis of ion channels, which have been known to play a crucial role in the process of apoptosis (Kondratskyi et al., 2015).
Ion channels are integral, membrane pore forming protein; tightly regulate ion flux and ionic concentration in the cell as well as in the intra cellular organelles and thereby which have been likely involved in normal physiological metabolism and modified expression of these channels account for numerous diseases (RamaKrishnan et al., 2016). Cells express plethora of distinct ion channels that can be classified, based on gating mechanism like voltage, ligand gated and temperature dependent and based on the types of ions that conduct it can be differentiated into Calcium, potassium channels and sodium channels to name few. Thus, voltage gated ion channels, in turn classified into voltage gated sodium channel (VGSC), Voltage Gated Calcium Channel (VGCC), Voltage Gated Potassium Channel (VGKC) (RamaKrishnan et al., 2016). In addition to this, special subtypes like Acid Sensing Ion channel (ASIC) and Hyper polarization activated Cyclic nucleotide gated channel (HCN) also exist which pursue peculiar mechanism of activation.
Growing body of evidence suggest that increased expression of VGCC and ensued enhanced calcium influx have been likely contributed in the pancreatic cell apoptosis which is attenuated by inhibiting the calcium influx by using the specific blocker of VGCC (Zaitsev et al., 2001). The paramount importance of VGSC and HCN, latter by mediating calcium influx in the neuronal cell apoptosis has been demonstrated previously (Kondratskyi et al., 2015, Banasiak et al., 2004); however there is no convincing report of these 2 ion channels in the pancreatic cell apoptosis. Thus, we have selected VGSC and HCN channels, to study the differential expression in the pancreatic cell apoptosis which is major pathogenesis of Type 1 Diabetes (T1D).
Materials and Methods
Experimental design
This study was carried out in accordance with the protocol approval of Animal ethical committee from Sathyabama University, Chennai, India. All the BALB/c mice, weighed 25-30 g were housed in 12 hours light and 12 hours dark period and fed with rodent pellets, water ad libitum. Freshly prepared alloxan monohydrate (purchased from Sigma Aldrich) in 0.9% saline was used for single intraperitoneal injection for inducing T1D. Mice were divided into 3 groups, each with 8 mice that encompasses, inducing with 0.1 μl of physiological saline, inducing with Alloxan monohydrate (186.9 mg/kg of weight) for 12 hours and 24 hours. All the induced mice were allowed to drink 5% glucose solution to prevent initial hypoglycemia. Blood glucose level was measured with glucometer Dr. MorepenGluco One model: BG-03. Blood glucose level of above 200mg/dl was considered as diabetic and used for further study. For plasma isolation, blood was collected from cardiac puncture of control, T1D mice and followed by centrifugation at 1800 rpm for 20 minutes, which was stored at -80°C for further use.
Pancreas isolation from BALB/c mice
Pancreas was extracted from control and diabetic BALB/c mice by following the protocols as mentioned previously (Zongyi et al., 2017). Briefly, dissected pancreas was kept in a digestion buffer, containing collagenase at 37°C in a shaker incubator. After 20 minutes, digestion was stopped by adding BSA containing solution and subsequently centrifuged at 1000 rpm for 2 min. Pellet was mixed with ficoll solution and then centrifuged. Islet was obtained as pellet and washed thoroughly with PBS and used for future experiments.
Cell culture and treatment of βTC6 cells, Cell viability
Beta TC-6 was cultured in the high glucose DMEM medium (10%FBS) with 2 mM glutamine and 500 μl sodium pyruvate (purchased from Lonza) at 37°C, in an incubator with the atmosphere of 5% CO2. Cell number was counted with a hemocytometer and sub cultured in the ratio of 1:2 or 1:3 based on the confluency. To mimic the pancreatic apoptotic condition, cells were treated with control plasma (CP), diabetic plasma (DP) and alloxan for 6.12 and 24 hours (Kikumoto et al., 2010; Jayasena et al., 2015). MTT (3-(4,5 dimethythiazol- 2-yl)-2,5-diphenyl tetrazolium bromide) (purchased from Calbiochem) colorimetric assay at the concentration of 5mg/ml was used for analyzing the cell viability (Yedjou et al., 2007) in βTC6 cells. Briefly, after the respective treatment, medium was replaced with MTT solution, then incubated for 3-4 hours at 37°C at CO2 incubator. The insoluble formazan was dissolved by adding of IPA (solubilisation solution) and the absorbance was measured with BioRad microplate reader (Model 680) at 570 nm. The rate of cell viability was calculated by using the formula % of cell survival = (mean absorbance of experimental/mean absorbance of control x 100).
Reverse transcription and gene expression study
Trizol (from Takara) and chloroform (Sigma Aldrich) solution was used for isolating RNA from pancreatic islets and βTC6 cells as recommended previously (Ikeda et al., 2015). Finally, RNA was dissolved in nuclease free water and stored at -80°C. RNA quality and concentrations were quantified with the help of Thermoscientific NanoDrop 1000. C-DNA was prepared using Revertid Reverse transcriptase and random hexamer and subsequently real time PCR was carried out using 1X Sybr green master mix. All these solutions acquired from Thermo fisher Scientific. Reaction solution consisting of master mix, cDNA, forward and reverse primer (Table 1) was prepared. Quantitative Real time PCR was carried out with Eppendorf MasterCycler RealPlex.2. Cycling parameters, 95°C for 2 minutes, 95°C for 30 sec, annealing for 1.3 minutes and extension at 72°C for 1 min were used and cycles have been repeated for 35x cycles. These data were normalized with two internal control genes, such as GAPDH and β actin.
| S. No | Gene name | Sense strand | Anti sense strand |
|---|---|---|---|
| 1. | scna | AGACCGCGAGTCTGTCGT | GGGTTTGCCATCAAGCTG |
| 2. | hcn | AGGACTTCCCCGATGACTG | TTTCCCCAGGAGTTGTTCAC |
Table 1: List of primers used for gene expression study.
Statistical analysis
Statistical analysis was performed using Prism and Sigma stat software. One way anova analysis with Tukey’s test was performed using sigma stat (version 3.5). Data showed as ± SEM values and statistically significant p value was mentioned in each results.
Results
Blood glucose measurement
Type 1 Diabetes as evidenced by increased amount of the blood glucose level, on account of the complete destruction of pancreatic beta cells. The major findings for this destruction are the pancreatic beta cells misrecognized as foreign antigen by immune system (Wang et al., 2010). Thus, blood glucose level was measured from the tail blood samples of control and diabetic BALB/c mice. We have observed more than 200mg/dl of blood glucose level in 12 hours alloxan treated mice and 24 hours alloxan treated mice exhibited blood glucose level of above 250 mg/dl (Table 2). These mice were considered as diabetic and used for the further gene expression analysis.
| S. No | Condition | Blood glucose(mg/dl) |
|---|---|---|
| 1 | Saline treated mice | 147- 190 |
| 2 | 12 hrs Alloxan treated mice | 218-383 |
| 3 | 24 hrs Alloxan treated mice | 265-483 |
Table 2: Blood glucose level of saline and Alloxan treated BALB/c mice
Cell viability and gene expression of apoptotic genes
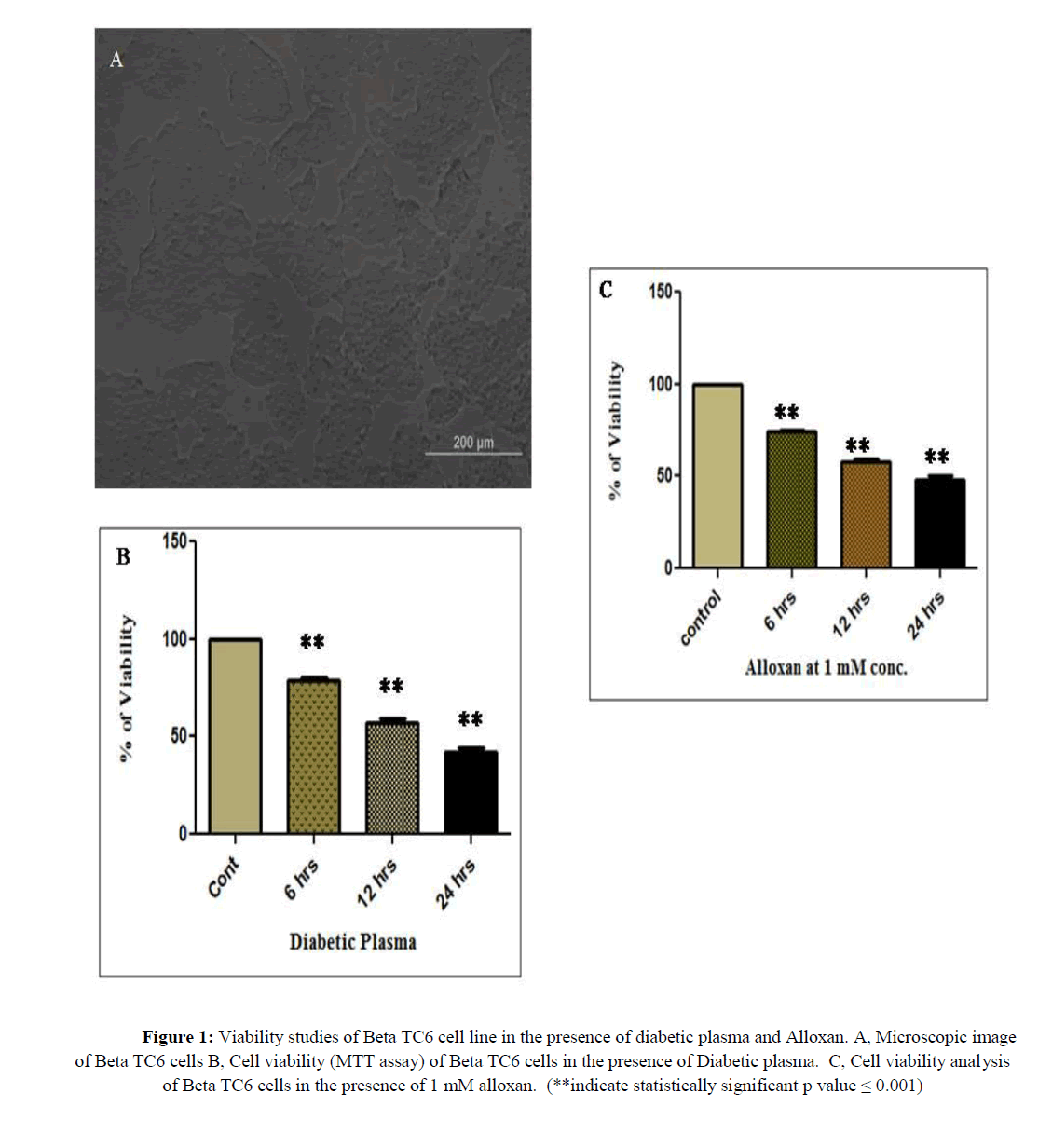
We have investigated preliminary analysis of βTC6 cell viability in the administration of various concentrations (like 1/4th and 1/2nd volume of the medium) of CP, 12 hrs DP and 24 hrs DP for 6, 12 and 24 hours treatment. Among that we have optimized DP from 24 hours alloxan treated BALB/c mice and CP from control mice at the concentration of 1/4th volume for our studies based on the cell viability and statistical analysis.
As we wanted to study the ion channel expression in pre apoptotic condition, we have chosen 6 and 12 hours as our experimental duration time and neglected 24 hours treatment, in the latter we have observed less than 50% of cell viability. Similarly, we have selected 1 mM of alloxan for our gene expression study from rest of concentrations like 0.2, 0.5 and 5 mM. Table 3 and figure 1 illustrated the results of these cell viability assay, 78 and 57% of cell viability was acquired in βTC6 cells in response to 6 and 12 hours treatment of DP (p ≤ 0.001) as well as 74% and 57% of cell viability was obtained in βTC6 cells in response to the alloxan treatment for 6 and 12 hours.
| Treatment | Set 1 |
% of viability | Set 2 |
% of viability | Set 3 |
% of viability | Set 4 |
% of viability | Set 5 |
% of viability | Set 6 |
% of viability | Avg. viability |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cont 6 hr | 0.515 | 100.00 | 0.45 | 100.00 | 0.69 | 100.00 | 0.68 | 100.00 | 0.49 | 100.00 | 0.51 | 100.00 | 100.00 |
| ALX 6 hr | 0.389 | 75.53 | 0.34 | 75.72 | 0.50 | 72.20 | 0.49 | 72.53 | 0.38 | 77.21 | 0.36 | 70.96 | 74.02 |
| Cont 12 hr | 0.505 | 100.00 | 0.49 | 100.00 | 0.50 | 100.00 | 0.52 | 100.00 | 0.49 | 100.00 | 0.51 | 100.00 | 100.00 |
| ALX 12 hr | 0.312 | 61.78 | 0.30 | 62.22 | 0.28 | 57.17 | 0.29 | 55.15 | 0.29 | 59.34 | 0.26 | 49.90 | 57.59 |
| Cont 24 hr | 0.476 | 100.00 | 0.42 | 100.00 | 0.49 | 100.00 | 0.42 | 100.00 | 0.49 | 100.00 | 0.51 | 100.00 | 100.00 |
| ALX 24 hr | 0.224 | 47.06 | 0.24 | 56.16 | 0.25 | 51.02 | 0.22 | 52.39 | 0.21 | 43.53 | 0.19 | 36.45 | 47.77 |
| Cont 6 hr | 0.498 | 100.00 | 0.52 | 100.00 | 0.49 | 100.00 | 0.48 | 100.00 | 0.49 | 100.00 | 0.51 | 100.00 | 100.00 |
| DP 6 hr | 0.357 | 71.69 | 0.41 | 78.27 | 0.41 | 83.37 | 0.39 | 81.93 | 0.39 | 79.47 | 0.40 | 77.73 | 78.74 |
| CP 6 hr | 0.421 | 84.54 | 0.43 | 83.46 | 0.39 | 79.47 | 0.40 | 82.98 | 0.42 | 85.22 | 0.43 | 83.20 | 83.14 |
| Cont 12 hr | 0.432 | 100.00 | 0.49 | 100.00 | 0.43 | 100.00 | 0.52 | 100.00 | 0.46 | 100.00 | 0.45 | 100.00 | 100.00 |
| DP 12 hr | 0.285 | 65.97 | 0.29 | 60.37 | 0.23 | 54.67 | 0.25 | 47.40 | 0.26 | 55.94 | 0.26 | 58.41 | 57.13 |
| CP 12 hr | 0.326 | 75.46 | 0.37 | 74.95 | 0.34 | 78.27 | 0.39 | 74.57 | 0.36 | 77.54 | 0.34 | 76.11 | 76.15 |
| Cont 24 hr | 0.498 | 100.00 | 0.52 | 100.00 | 0.49 | 100.00 | 0.48 | 100.00 | 0.47 | 100.00 | 0.48 | 100.00 | 100.00 |
| DP 24 hr | 0.179 | 35.94 | 0.23 | 43.46 | 0.22 | 44.35 | 0.22 | 46.85 | 0.28 | 59.20 | 0.26 | 53.33 | 47.19 |
| CP 24 hr | 0.356 | 71.49 | 0.33 | 63.27 | 0.38 | 78.85 | 0.35 | 73.32 | 0.29 | 60.25 | 0.31 | 65.42 | 68.77 |
Table 3: Cell Viability assay of DP and alloxan treated Beta TC6 cells
Figure 1: Viability studies of Beta TC6 cell line in the presence of diabetic plasma and Alloxan. A, Microscopic image of Beta TC6 cells B, Cell viability (MTT assay) of Beta TC6 cells in the presence of Diabetic plasma. C, Cell viability analysis of Beta TC6 cells in the presence of 1 mM alloxan. (**indicate statistically significant p value = 0.001)
Apoptosis inducing ability of DP and alloxan in βTC6 cells have been further corroborated by gene expression study of pro, anti-apoptotic genes like Bax and Bcl-2 (Chueh et al., 2012). Apoptotic gene like Bax has been up regulated in 12 and 24 hours alloxan treated pancreatic islets from BALB/c mice (data not shown) as well as in vitro 6 and 12 hours of DP and alloxan treated βTC6 cells. Similarly anti apoptotic gene, BCl2 have been down regulated in all the above conditions.
Relative gene expression of VGSC in the apoptotic pancreatic islets from T1D mice and apoptotic βTC6 cells
Previous studies have implied that increased expression of sodium channel and thereby facilitated sodium influx likely involved in the apoptotic process. Banasiak et al. reported that oxygen deprivation in neuronal cells activates VGSC and that mediate caspase 3 dependent apoptosis (Kondratskyi et al., 2015, Banasiak et al., 2004). We have analyzed meta data of Hansen et al., 2012 and Chaparro et al., 2006 by in-silico studies and observed down regulated expression of sodium channel alpha subunit in apoptotic pancreatic cells like IL-1β treated INS-1 cells and diabetic sensitive NOD SCID mice (under publication). In this present study, we have examined the expression of VGSC-α (scnα) subunit in the pancreatic islets of alloxan treated mice and apoptotic βTC6 cells in response to alloxan and DP administration. Alloxan treatment for 12 and 24 hours in mice upregulated and down regulated scnα expression to 5.5 fold with a p value of 0.009 and 3.6 fold with statistically significant p value (p ≤ 0.001), respectively. Similarly, in vitro 6 and 12 hours treatment of DP up regulated and down regulated scna expression to 3.0 and 3.5 fold (p ≤ 0.001), however both 6 and 12 hours treatment of alloxan upregulated, scna gene to 19 fold (p=0.001) and 18 fold (p=0.010) in βTC6 cells. Thus up regulated expression of VGSC observed in the early stage of in vitro and in vivo apoptotic pancreatic cells (Figure 2).
Figure 2: Gene expression study of VGSC (scna) gene in apoptotic pancreatic cells. A, Relative gene expression of scna gene in pancreatic islets from 12 and 24 hours alloxan treated diabetic mice. B, Relative gene expression of scna gene in 6 and 12 hours diabetic plasma treated apoptotic Beta TC6 cells. C, Relative gene expression of scna gene in 6 and 12 hours 1 mM Alloxan treated apoptotic Beta TC6 cells. (** indicate statistically significant p value = 0.001).
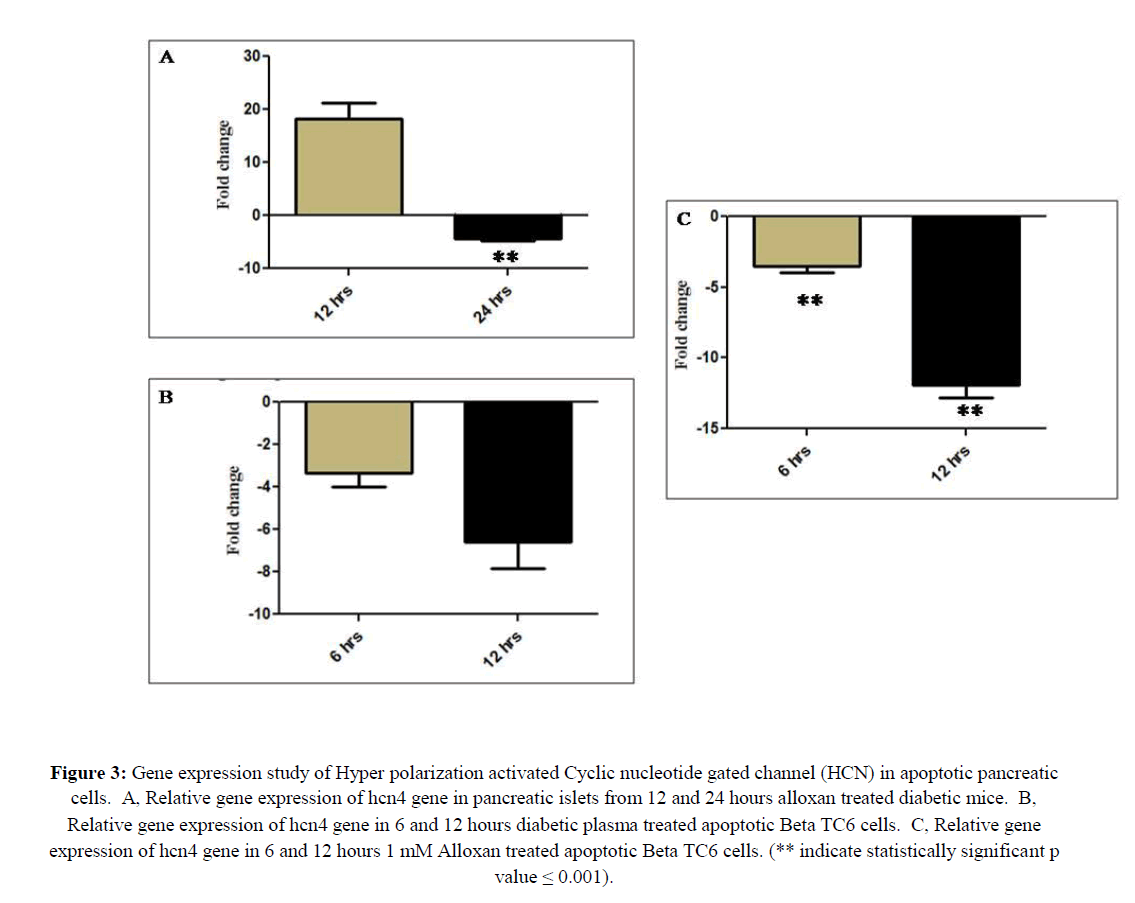
Relative gene expression of HCN channel in the apoptotic pancreatic islets from T1D mice and apoptotic βTC6 cells
HCN channel also known as pace maker channels, has been well characterized in the neuronal and cardiac function like generating rhythmic activity, neuronal excitability, synaptic transmission and memory related process (Benarroch, 2013). The contribution of this channel in the apoptosis is poorly studied. It has been reported by two different research groups that upregulation of this channel in ganglion neuron account for neuronal apoptosis as well as siRNA mediated down regulation of this channel in lung carcinoma cells attenuated apoptosis (Shen et al., 2018; Norberg et al., 2010). Thus, it was tempting to study the expression of the same in pancreatic apoptosis. This hcn4channel gene has been up and down regulated to 16 (p=0.012) and 4.3 fold (p ≤ 0.001), respectively, in apoptotic pancreatic islets of 12 and 24 hours alloxan treated mice. And also 6 and 12 hours treatment of DP to the pancreatic β cell line βTC6 down regulated hcn4 gene to 3.3 fold (p=0.003) and 6.5 fold (p=0.004), respectively. Similarly 6 and 12 hours in vitro treatment of alloxan down regulated hcn4 gene to 3.5 fold and 11 fold (p ≤ 0.001) (Figure 3).
Figure 3: Gene expression study of Hyper polarization activated Cyclic nucleotide gated channel (HCN) in apoptotic pancreatic cells. A, Relative gene expression of hcn4 gene in pancreatic islets from 12 and 24 hours alloxan treated diabetic mice. B, Relative gene expression of hcn4 gene in 6 and 12 hours diabetic plasma treated apoptotic Beta TC6 cells. C, Relative gene expression of hcn4 gene in 6 and 12 hours 1 mM Alloxan treated apoptotic Beta TC6 cells. (** indicate statistically significant p value = 0.001).
Discussion
Pancreatic beta cell apoptosis is arbitrated by increasing intra cellular calcium concentration, which activate the various downstream signaling pathway like activating ERK (Extracellular Signal Regulated Kinase) pathway and/or calcium dependent cell death enzyme like caspases, endonucleases, calpain and calcineurin (Ramadan et al., 2011; Kim et al., 2002) to trigger apoptosis. For instance, calcineurin dephosphorylates BCl2 family member, Bad for inducing pro apoptotic effect on the cell (Wang et al., 1999).
In this study, alloxan induced T1D in BALB/ mice have been corroborated by examining the blood glucose level of more than 200mg/dl in all 12 hours and above 250mg/dl of all 24 hours alloxan administrated mice. And also pancreatic apoptotic was confirmed by the gene expression study of pro and anti-apoptotic genes (Chueh et al., 2012) in the pancreatic islets of alloxan treated mice. Similarly, apoptosis inducing ability of DP and alloxan treatment has been analyzed by colorimetric assay, MTT. We have identified reduced cell viability in 6, 12 and 24 hours DP and alloxan treated βTC6 cells, from that we have chosen 6 and 12 hours for our study to study the ion channel gene expression in pro apoptotic condition.
By taking the account of evidence from previous studies, it has been investigated that increased expression of VGCC likely contributed in T1D serum as well as cytokine induced pancreatic cell apoptosis by boosting calcium influx into the cell (Zaitsev et al., 2001; Juntti-Berggren et al., 1993; Wang et al., 1999). Nevertheless, VGSC and HCN channel driven apoptosis in pancreatic cells have long been remain unknown, thus which was the main objective of this study.
VGSC composed of pore forming α subunit, associated with 1 or 2, identical or different auxiliary β subunit, latter control kinetics and voltage gating of the channel, together these subunits results in the formation of distinct variant of VGSC (Goldin et al., 2000). Sodium channel has been well known for inducing rapid, initial depolarization phase of action potential (AP) in neuronal cell membrane, mediating fast inactivation within 1-2 ms and thereby responsible for repolarization phase and that followed by termination of AP. Mutation in this genes account for numerous diseases collectively termed sodium channelopathies, like Brugada syndrome, skeletal myopathies to name few (Roger et al., 2015). Increased intracellular sodium concentration has been observed in UV and strastosporine induced apoptosis which have been identified with chromatin condensation, DNA fragmentation, poly (ADP-ribose) polymerase cleavage and loss of mitochondrial membrane potential (Arrebola et al., 2006; Arrebola et al., 2005). These increased sodium concentration funneled through the sodium channels and indeed, upregulated expression of VGSC has been well observed in Staurosporine treated Xenopus oocytes as well as in the oxygen deprivation induced apoptotic neuronal cell (Banasiak et al., 2004; Englund et al., 2014). In this present investigation, we have observed upregulated expression of VGSC in the early phase of vivo alloxan treated pancreatic islets from BALB/c mice as well as in vitro DP and alloxan treated βTC6 cells. Activated VGSC cause sodium influx that attribute for membrane depolarization such as flooding cation into the cells, cause ionic imbalance which is a primary requisite for stimulating early phase of apoptosis in the pancreatic cells.
Oxygen deprived neuronal cell death attenuated in the presence of tetrodotoxin, VGSC blocker, which is observed with declined DNA fragmentation and Caspase 3 activation (McIlwain et al., 2013). Similarly, veratridine induced neuronal cell apoptosis and toxicity, triggered by ROS production have been attenuated by the addition of neuroprotective agent AM-36 which confers bifunctional pharmacological activities like anti-oxidant and sodium channel blocker activity (Callaway et al., 2001). Typically, apoptosis is evidenced by change in cell volume which is executed by the activity of VGSC, inhibition of the same by Saxitoxin prevented the change in cell size and apoptosis (Nolte et al., 2004; Bortner et al., 2003). Similarly Yang et al. proposed that Carbamazine, use dependent sodium channel blocker, modulates cytosolic, ER and mitochondrial Calcium and diminishes pro apoptotic and ER stress mechanism of cytokines in pancreatic islets (Yang et al., 2014). Thus, it is possible to attenuate pancreatic apoptosis by the addition of VGSC specific blocker at the initial stage of pancreatic apoptosis.
HCN channel, stimulated by membrane hyperpolarization, permeable to cations, activated by direct interaction of cyclic nucleotide, mostly cAMP (cyclic Adenosine Monophosphate). The cation current on account of the influx of cation through this channel facilitates the membrane to depolarized state (Benarroch, 2013). These HCN channels have been differentiated into 4 subtypes, HCN1-4 based on the gating mechanism and sensitivity to cAMP.
Phan et al. demonstrated by meta-analysis that these channels are up and down regulated in different types of cancer, for instance, HCN4 upregulated in lung, kidney, prostate, ovarian, bladder and thyroid cancer and the same has been down regulated in breast, bladder and exophageal cancer (Phan et al., 2017). Numerous reports demonstrated that several cardiac diseases precipitated by the impaired regulation of this channel, for instance mutation in the hcn4 gene provoke cardiac structural abnormalities like bradycardia and sick sinus syndrome (DiFrancesco, 2015; Zhao et al., 2016). Among the 4 types of HCN channels, HCN2 and HCN4 are mainly implicated in the calcium flux at negative membrane potential, latter likely attributed for inducing apoptosis (Benarroch, 2013; Yu et al., 2004; Michels et al., 2008). And contribution of HCN4 in the apoptosis has been unappreciated, thus we have selected HCN4 channel for our study. Shen et al. elucidated that upregulated expression of HCN channels in Spiral Ganglion Neuron (SGN) of old mice contributed for the age related changes of AP (Action Potential), age related degradation of SGN as well as inducing SGN apoptosis. SGN apoptosis in old mice responsible for the presence of reduced number of SGN as well as age dependent hearing loss (Shen et al., 2018). We have observed by RT PCR analysis that excluding in the apoptotic pancreatic islets obtained from 12 hours alloxan treated mice, rest of the apoptotic pancreatic cells exhibited down regulated expression of this hcn4 gene.
Moreover, PKC inhibitors induce cell death and chromatin condensation in NSCLC and primary cultures of rat cortical neuronal cells by mediating calcium entry through HCN2 channels and liberation of AIF (Apoptotic Inducing Factor) from mitochondrial inner membrane to nucleus which is the independent apoptotic pathway of caspase activation. siRNA mediated down regulation of this channel attenuates this PKC inhibitors effect on calcium entry, nuclear translocation of AIF, chromatin condensation as well as cell death (Norberg et al., 2010). We hypothesize here that membrane depolarization because of increased cytosolic calcium concentration in the apoptotic pancreatic cells down regulate this HCN channel expression in the apoptotic conditions. Though, it has been reported that HCN currents are stimulated and inhibited by membrane hyperpolarization and depolarization, respectively (Norberg et al., 2010), there is no current report states the differential expression of this channel by membrane depolarization. The expression of this channel differs based on the subtype and distribution in sub cellular compartments, which is also modulated by phosphotidyl inositol triphosphate, cAMP to name few (Benarroch, 2013). Zong et al. described that channel gating is affected by tyrosine phosphorylation at the C-terminus region in response to the binding of Src kinase (Zong et al., 2005). However, there is much to learn about this channel in the pancreatic apoptosis mechanism.
Study Limitation
This study has been carried out in the in-vitro and in-vivo models of pancreatic apoptosis. Studies on the post transcriptional changes as well as differential expression of the proteins involved would shed further light on the underlying molecular and cellular mechanisms. While we have studied the difference in expression pattern of ion channel genes, this complemented with clinical studies may help understand the pancreatic apoptosis pathway better paving way for novel treatment modalities for Type 1 Diabetes.
Conclusion
We have observed by RT PCR analysis, up regulated expression of VGSC in the in vivo 12 hours alloxan treated pancreatic islets as well as in vitro 6 hours DP and alloxan treated βTC6 cells which is responsible for the early depolarizing phase of apoptosis. Thus specific blocker of this channel in the initial stage of pancreatic apoptosis could assist the beta cell survivability in T1D conditions. Moreover down regulated expression of HCN channel noticed in pancreatic islets of 24 hours alloxan treated mice and 6 and 12 hours of DP and alloxan treated βTC6 cells. Thus further study in this channel will delineate the underlying mechanism. However still there is a long way for using these ion channel modulator in T1D therapeutics. Thus, this present work will be pilot study for sodium and HCN channel mediated treatment in T1D.
Acknowledgment
AMR acknowledges Anna University for providing Anna Centenary Research fellowship during this study.
Conflict of Interest
All authors declare there is no conflict of interest.
About the Authors
Corresponding Author
Kavitha Sankaranarayanan
AU-KBC Research Centre, Madras Institute of Technology, Anna University, Chrompet, Chennai, India
- Email:
- skavitham@yahoo.com
References
- Arrebola F, Fernández-Segura E, Campos A, Crespo PV, et al. (2006) Changes in intracellular electrolyte concentrations during apoptosis induced by UV irradiation of human myeloblastic cells. Am J Physiol Cell Physiol 290: C638-C649. https://doi.org/10.1152/ajpcell.00364.2005
- Arrebola F, Zabiti S, Cañizares FJ, Cubero MA, et al. (2005) Changes in intracellular sodium, chlorine, and potassium concentrations in staurosporine-induced apoptosis. J Cell Physiol 204: 500-507. https://doi.org/10.1002/jcp.20306
- Banasiak KJ, Burenkova O, Haddad GG (2004) Activation of voltage-sensitive sodium channels during oxygen deprivation leads to apoptotic neuronal death. Neuroscience 126: 31-44. https://doi.org/10.1016/s0306-4522(03)00425-1
- Benarroch EE (2013) HCN channels: function and clinical implications. Neurology 80: 304-310. https://doi.org/10.1212/wnl.0b013e31827dec42
- Bortner CD and Cidlowski JA (2003) Uncoupling cell shrinkage from apoptosis reveals that Na+ influx is required for volume loss during programmed cell death. J Biol Chem 278: 39176-39184. https://doi.org/10.1074/jbc.m303516200
- Callaway JK, Beart PM, Jarrott B, Giardina SF (2001) Incorporation of sodium channel blocking and free radical scavenging activities into a single drug, AM-36, results in profound inhibition of neuronal apoptosis. Br J Pharmacol 132: 1691-1698. https://doi.org/10.1038/sj.bjp.0704018
- Chaparro RJ, Konigshofer Y, Beilhack GF, Shizuru JA, et al. (2006) Nonobese diabetic mice express aspects of both type 1 and type 2 diabetes. Proc Natl Acad Sci 103: 12475-12480. https://doi.org/10.1073/pnas.0604317103
- Chueh WH and Lin JY (2012) Berberine, an isoquinoline alkaloid, inhibits streptozotocin-induced apoptosis in mouse pancreatic islets through down-regulating Bax/Bcl-2 gene expression ratio. Food Chem 132: 252-260. https://doi.org/10.1016/j.foodchem.2011.10.065
- DiFrancesco D (2015) HCN4, Sinus Bradycardia and Atrial Fibrillation. Arrhythm Electrophysiol Rev 4: 9-13. https://doi.org/10.15420/aer.2015.4.1.1
- Englund UH, Gertow J, Kågedal K, Elinder F (2014) A voltage dependent non-inactivating Na+ channel activated during apoptosis in Xenopus oocytes. PLoS One 9: e88381. https://doi.org/10.1371/journal.pone.0088381
- Eizirik DL and Mandrup-Poulsen T (2001) A choice of death--the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia 44: 2115-2133. https://doi.org/10.1007/s001250100021
- Goldin AL, Barchi RL, Caldwell JH, Hofmann F, et al. (2000) Nomenclature of voltage-gated sodium channels. Neuron 28: 365-368. https://doi.org/10.1016/s0896-6273(00)00116-1
- Hansen JB, Tonnesen MF, Madsen AN, Hagedorn PH, et al. (2012) Divalent metal transporter 1 regulates iron-mediated ROS and pancreatic β cell fate in response to cytokines. Cell Metab 16: 449-461. https://doi.org/10.1016/j.cmet.2012.09.001
- Ikeda K, Tomimoto S, Tsuchiya S, Hamagami KI, et al. (2015) Comparative gene expression profiles in pancreatic islets associated with agouti yellow mutation and PACAP overexpression in mice. Biochem Biophys Rep 2: 179-183. https://doi.org/10.1016/j.bbrep.2015.06.006
- Jayasena T, Poljak A, Braidy N, Smythe G, et al. (2015) Upregulation of glycolytic enzymes, mitochondrial dysfunction and increased cytotoxicity in glial cells treated with Alzheimer's disease plasma. PLoS One 10: e0116092. https://doi.org/10.1371/journal.pone.0116092
- Juntti-Berggren L, Larsson O, Rorsman P, Ammälä C, et al. (1993) Increased activity of L-type Ca2+ channels exposed to serum from patients with type I diabetes. Science 261: 86-90. https://doi.org/10.1126/science.7686306
- Kikumoto Y, Sugiyama H, Inoue T, Morinaga H, et al. (2010) Sensitization to alloxan-induced diabetes and pancreatic cell apoptosis in acatalasemic mice. Biochim Biophys Acta 1802: 240-246. https://doi.org/10.1016/j.bbadis.2009.10.009
- Kim MJ, Jo DG, Hong GS, Kim BJ, et al. (2002) Calpain-dependent cleavage of cain/cabin1 activates calcineurin to mediate calcium-triggered cell death. Proc Natl Acad Sci 99: 9870-9875. https://doi.org/10.1073/pnas.152336999
- Kondratskyi A, Kondratska K, Skryma R, Prevarskaya N (2015) Ion channels in the regulation of apoptosis. Biochim Biophys Acta 1848: 2532-2546. https://doi.org/10.1016/j.bbamem.2014.10.030
- McIlwain DR, Berger T, MakTW (2013) Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 5: a008656-008656. https://doi.org/10.1101/cshperspect.a008656
- Michels G, Brandt MC, Zagidullin N, Khan IF, et al. (2008) Direct evidence for calcium conductance of hyperpolarization-activated cyclic nucleotide-gated channels and human native If at physiological calcium concentrations. Cardiovasc Res 78: 466-475. https://doi.org/10.1093/cvr/cvn032
- Nolte F, Friedrich O, Rojewski M, Fink RH, et al. (2004) Depolarisation of the plasma membrane in the arsenic trioxide (As2O3)-and anti-CD95-induced apoptosis in myeloid cells. FEBS Lett 578: 85-89. https://doi.org/10.1016/j.febslet.2004.10.075
- Norberg E, Karlsson M, Korenovska O, Szydlowski S, et al. (2010) Critical role for hyperpolarization-activated cyclic nucleotide-gated channel 2 in the AIF-mediated apoptosis. EMBO J 29: 3869-3878. https://doi.org/10.1038/emboj.2010.253
- Phan NN, Huynh T, Lin YC (2017) Hyperpolarization activated cyclic nucleotide gated gene signatures and poor clinical outcome of cancer patient. Transl Cancer Res 6: 698-708. https://doi.org/10.21037/tcr.2017.07.22
- Pirot P, Cardozo AK, Eizirik DL (2008) Mediators and mechanisms of pancreatic beta-cell death in type 1 diabetes. Arq Bras Endocrinol Metabol 52: 156-165. https://doi.org/10.1590/s0004-27302008000200003
- Ramadan JW, Steiner SR, O'Neill CM, Nunemaker CS (2011) The central role of calcium in the effects of cytokines on beta-cell function: implications for type 1 and type 2 diabetes. Cell Calcium 50: 481-490. https://doi.org/10.1016/j.ceca.2011.08.005
- RamaKrishnan AM and Sankaranarayanan K (2016) Understanding autoimmunity: The ion channel perspective. Autoimmun Rev 15: 585-620. https://doi.org/10.1016/j.autrev.2016.02.004
- Roger S, Gillet L, Le Guennec JY, Besson P (2015) Voltage-gated sodium channels and cancer: is excitability their primary role?. Front Pharmacol 6: 152. https://doi.org/10.3389/fphar.2015.00152
- Shen H, Liu W, Geng Q, Li H, et al. (2018) Age-dependent up-regulation of HCN channels in spiral ganglion neurons coincide with hearing loss in mice. Front Aging Neurosci 10: 353. https://doi.org/10.3389/fnagi.2018.00353
- Yang YH, Vilin YY, Roberge M, Kurata HT, et al. (2014) Multiparameter screening reveals a role for Na+ channels in cytokine-induced β-cell death. Mol Endocrinol 28: 406-417. https://doi.org/10.1210/me.2013-1257
- Yedjou CG and Tchounwou PB (2007) In-vitro cytotoxic and genotoxic effects of arsenic trioxide on human leukemia (HL-60) cells using the MTT and alkaline single cell gel electrophoresis (Comet) assays. Mol Cell Biochem 301: 123-130. https://doi.org/10.1007/s11010-006-9403-4
- Yu X, Duan KL, Shang CF, Yu HG, et al. (2017) Calcium influx through hyperpolarization-activated cation channels (I(h) channels) contributes to activity-evoked neuronal secretion. Proc Natl Acad Sci 101: 1051-1056. https://doi.org/10.1073/pnas.0305167101
- Wang L, Bhattacharjee A, Zuo Z, Hu F, et al. (1999) A low voltage-activated Ca2+ current mediates cytokine-induced pancreatic beta-cell death. Endocrinology 140: 1200-1204. https://doi.org/10.1210/endo.140.3.6556
- Wang C, Guan Y, Yang J (2010) Cytokines in the Progression of Pancreatic β-Cell Dysfunction. Int J Endocrinol. 2010: 1-10.https://doi.org/10.1155/2010/515136
- Wang HG, Pathan N, Ethell IM, Krajewski S, et al. (1999) Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science 284: 339-343. https://doi.org/10.1126/science.284.5412.339
- Zaitsev SV, Appelskog IB, Kapelioukh IL, Yang SN, et al. (2001) Imidazoline compounds protect against interleukin 1beta-induced beta-cell apoptosis. Diabetes 50: S70-S76. https://doi.org/10.2337/diabetes.50.2007.s70
- Zhao X and Gu T (2016) Dysfunctional Hyperpolarization-Activated Cyclic Nucleotide-gated Ion Channels in Cardiac Diseases. Braz J Cardiovasc Surg 31: 203-206. https://doi.org/10.5935/1678-9741.20160030
- Zongyi Y, Funian Z, Hao L, Ying C, et al. (2017) A rapid, efficient, and economic device and method for the isolation and purification of mouse islet cells. PLoS One 12: e0171618. https://doi.org/10.1371/journal.pone.0171618
- Zong X, Eckert C, Yuan H, Wahl-Schott C, et al. (2005) A novel mechanism of modulation of hyperpolarization-activated cyclic nucleotide-gated channels by Src kinase. J Biol Chem, 280: 34224- 34232. https://doi.org/10.1074/jbc.m506544200
Keywords:
Download:
Full PDF- Share This