Genetic diversity among accessions of Capsicum annuum L. through morphoagronomic characters
Received: January 14, 2018
Accepted: February 24, 2018
Published: February 26, 2018
Genet.Mol.Res. 17(1): gmr16039883
DOI: 10.4238/gmr16039883
Abstract
Genetic variability among pepper genotypes is essential in obtaining hybrid combinations with greater heterotic effect and in obtaining superior strains. This work’s goal was to evaluate the genetic diversity between accessions of Capsicum annuum L., indicating the selection of promising individuals for ornamental purposes. The experiment was carried out in at the Plant Biotechnology Laboratory of the Center of Agricultural Sciences, Federal University of Paraíba. The experiment was conducted in a completely randomized design with 16 treatments and eight replications. The plants were evaluated for 28 morphoagronomic traits. Data were submitted to analysis of variance and grouped according to Scott and Knott's test at 1% probability. The Tocher grouping was performed based on Mahalanobis distance and analysis of canonical variables were performed with graphical dispersion of the accessions. All variables were significant by the F test (p = 0.01) and presented high heritability and a CVg/CVe ratio higher than 1.0 for most traits, indicating genetic divergence between accessions. In keeping with the Scott-Knott's test (p = 0.01), the accessions were grouped into two to eight classes, varying according to the character. The of Tocher optimization method separated the accessions into five distinct groups. There is phenotypic divergence between the accessions of Capsicum annuum L. which can be used in ornamental peppers’ breeding programs. Only the number of stamens trait presented a heritability value (65.81%) lower than 70%. The characters that most contributed to divergence among the accessions were fresh fruit mass, stem diameter, widest fruit diameter and fruit weight. The accessions UFPB001, UFPB004, UFPB45, UFPB77.3, UFPB099, UFPB134, UFPB390 and Calypso are designated as potential accessions for ideal ornamental pepper plant with vigorous seedling, small port, large flowers and small fruits. Ornamental pepper accessions with larger anthers are indicated for selection, for facilitating the breeder’s work during flower emasculations for crossings.
https://sporbahisleri.blogaaja.fi http://sporbahisleri.parsiblog.com https://spor-bahisleri.jimdosite.com https://sporbahisleri.edublogs.org https://sporbahisleri.websites.co.in https://sporbahisleri.podia.com https://sporbahisleri7.wordpress.com https://sporbahisleri.jigsy.com https://niwn-chroiaty-mcieung.yolasite.com https://spor-bahisleri.mywebselfsite.net https://sporbahisleri.mystrikingly.com https://sporbahisleri.splashthat.com https://sporbahisleri1.webnode.com.tr https://sporbahisleri.odoo.com http://sporbahisleri.creatorlink.net http://www.geocities.ws/sporbahisleri/ https://spor-s-site.thinkific.com https://artistecard.com/sporbahisleri https://sporbahisleri.estranky.cz https://spor-bahisleri.mozellosite.com https://651be6b563e56.site123.me https://betsitesiinceleme.blogspot.com https://sporbahisleri.hashnode.dev https://sporbahislerim.wixsite.com/spor-bahisleri https://sporbahislerix.weebly.com https://sites.google.com/view/betsiteleri https://codepen.io/sporbahisleri https://sporbahisleri.bcz.com https://www.smore.com/6rsb9
Introduction
Pepper plants (Capsicum annuum L.) belong to the family Solanaceae (Picksgill, 1997). They have edible fruits in addition to the ornamental potential (Stommel and Bosland, 2006; Rêgo et al. 2015a; Rêgo and Rêgo, 2016).
Throughout the world, the C. annuum species is the most cultivated and the most important economically, presenting sweet and pungent fruits (Wang and Bosland, 2006). The morphological diversity of this species when it comes to fruit color, shape, texture, size, aroma, and even pungency of the fruits, makes the market for pepper a diversified segment due to the amount of by-products that can be produced (Rêgo et al., 2011a).
The pepper agribusiness has great socioeconomic importance, since it encompasses family agriculture as well as small family-run, medium-sized and even multinational industries (Finger et al., 2012; Ulhoa et al., 2014; Rêgo et al. 2015a).
The market of ornamental plants in pots is increasing at a higher rate than the cut flower market. Among the potted ornamental plants, peppers (Capsicum spp.) are very popular in the retail markets due to consumers looking for new products (Rêgo et al., 2009).
Ornamental pepper offers a multitude of opportunities to develop unique cultivars, which can be commercialized in three ways: potted plants, garden plants and bouquets (Stommel and Bosland, 2006; Rêgo and Rêgo, 2016).
In order to use genetic resources efficiently, knowledge and organization in germplasm banks is essential, allowing the exploration of genetic variability. The effective use of variability in breeding programs depends on the information available about the population being studied (Rêgo et al., 2009; Rêgo et al., 2015b). The information about a germplasm collection’s variability helps to make the breeding process more efficient by describing the different accessions in the collection and their traits of interest (Neto et al., 2014; Costa et al. 2015; Neitzke et al. 2016). This will make possible to develop cultivars that meet the needs of the floriculture market, always looking for novelties and that present some characteristic that differentiates them in relation to the available cultivars.
The Universidade Federal da Paraíba (UFPB) maintains an active Germplasm Banks of pepper with 500 accessions, 290 hybrids and 1100 lineages in advanced generations. In the last two decades a breeding program of ornamental peppers has been developing at UFPB to evaluate and to select breeding lines and to promote the hybridization among the selected lines (Rêgo et al. 2015a; Rêgo and Rêgo, 2016).
When starting a breeding program, one of the critical points is choosing the parents to be used in the crosses in order to obtain a broad genetic base population in which the selection will act (Correa and Gonçalves, 2012).
The knowledge of diversity among accessions is fundamental since pepper breeding is based mainly on hybridization, generating segregating populations in order to obtain superior lines.
The genetic variability of morpho-agronomic traits, within and between accessions from the germplasm bank and of commercial varieties, has been the focus of many studies, e.g.: Rêgo et al., (2003), Sudré et al. (2005), Rêgo et al. (2011a,b), Nascimento et al. (2014), Nascimento et al. (2015) and Rêgo et al. (2015a,b).
Therefore, studies on genetic divergence are of great importance to breeding programs, as they allow the selection of superior parents to obtain hybrids with higher heterotic effect, which in turn allows the identification of plants with interesting characters in their segregating generations. Then, the objective of this work was to evaluate the genetic diversity among accessions of Capsicum annuum L. belong to Germaplasm Bank of Universidade Federal da Paraíba, in order to continue the breeding program of pepper with ornamental purposes.
Materials and Methods
The experiment was carried out in a greenhouse at the Plant Biotechnology Laboratory of the Center of Agricultural Sciences, Federal University of Paraíba (CCA-UFPB), Areia, State of Paraíba, Brazil.
Fifteen accessions of pepper plants (Capsicum annuum L.) belonging to CCA-UFPB’s Germplasm Bank were used: UFPB001, UFPB002, UFPB003, UFPB004, UFPB45, UFPB46, UFPB77.3, UFPB099, UFPB132, UFPB134, UFPB137, UFPB356, UFPB390, UFPB443, UFPB449, and one commercial cultivar of ornamental pepper, Calypso. The phenotypic description of the accessions is shown in Table 1.
| Accessions | PGH | LC | CC | FAS | FCI | FCM |
|---|---|---|---|---|---|---|
| UFPB001 | Erect | Light Green | White | Absent | Green | Yellow |
| UFPB002 | Erect | Green | White | Absent | Green | Red |
| UFPB003 | Erect | Green | White | Absent | Green | Orange |
| UFPB004 | Erect | Green | White | Absent | Green | Red |
| UFPB45 | Erect | Green and purple | Purple | Present | Dark Purple | Red |
| UFPB46 | Erect | Light Green | White | Present | Light Green | Red |
| UFPB77.3 | Erect | Variegated | Purple | Present | Orange-yellow | Red |
| UFPB099 | Erect | Green | White | Absent | Orange-yellow | Orange |
| UFPB132 | Erect | Light Green | Purple | Present | Purple | Red |
| UFPB134 | Intermediate | Green | White | Absent | Yellow | Orange |
| UFPB137 | Intermediate | Green | White | Absent | Yellow | Orange |
| UFPB356 | Intermediate | Dark Green | Purple | Absent | Green | Red |
| UFPB390 | Intermediate | Light Green | White | Absent | Orange-yellow | Red |
| UFPB443 | Erect | Dark Green | White | Absent | Yellow | Orange |
| UFPB449 | Erect | Dark Green | White | Absent | Yellow | Red |
| Calypso | Erect | Green | White | Absent | Green | Yellow |
PGH – Plant Growth Habit; LC – Leaf Color; CC – Corolla Color; FAS – Fruit Anthocyanin spots; FCI – Fruit color at intermediate stage and FCM – Fruit Color at mature stage.
Table 1. Description of six qualitative traits of the 16-ornamental pepper (Capsicum annuum L.) accessions used in this study. CCA/UFPB.
The accessions were self-pollinated and sowed in polystyrene trays of 128 cells filled with commercial substrate (Plantmax®) then, when they had at least six definitive leaves, they were transplanted into a 900 mL plastic vase containing the same substrate. Culture treatments were made according to the needs of culture.
Morphoagronomic characterization was based on the descriptor list suggested by IPGRI (1995). Twenty-eight quantitative traits were evaluated for seedling, plant, flower and fruit. The tools used to collect data were a digital caliper (Leetools® digital caliper) for measurements, a balance (Bel engineering®) for weight and a digital chlorophyllometer (ClorofiLOG - FALKER ®) for leaf chlorophyll content. Other quantitative data were taken by counting.
Seedling characterization was performed when they were transplanted to the final site, after 30 days of germination. The evaluated seedling traits were: seedling height (SH), cotyledons leaf length (CLL) and cotyledons leaf width (CLW). The plant characteristics evaluated were: plant height (PH), stem diameter (SD), height of first branching (HFB), canopy diameter (CD), leaf length (LL), leaf width (LW), as well as two physiological traits, Chlorophyll a (CLA) and Chlorophyll b (CLB).
Flower data were collected when they were fully opened. The variables analyzed were: flower diameter (FD), petal length (PL), number of petals (NP), number of stamens (NS), anther length (AL) and filament length (FL). Fruit data were collected when they were ripe and at first harvest. The evaluated characters were: number of fruits per plant (NFP), fruit weight (FW), fruit length (FL), widest fruit diameter (WFD), smallest fruit diameter (SFD), pedicel length (PDL), pericarp thickness (PT), placenta length (PLL), number seeds per fruit (NSF), fresh fruit mass (FFM) and dry matter content (DMC).
The experimental design was completely randomized with 16 treatments (accessions) with eight replicates. The data were submitted to analysis of variance, with a subsequent grouping of the means by the Scott-Knott test, at 1% probability. Estimates of heritability, genetic variance, and correlation between genetic and environmental coefficients were also calculated. The Tocher method (RAO, 1952), based on the generalized Mahalanobis distance and analysis of canonical variables with graphical dispersion of the genotypes, were used to analyze the genetic divergence. The relative importance of the variables was determined by the method described by Singh (1981) and by canonical variables. All analyses were performed with GENES computer software (Cruz, 2006).
Results
Differences among accessions were significant (p=0.01) for the evaluated seedling, plant, flower and fruit traits (Table 2).
| F.V. | Characters | ||||||
|---|---|---|---|---|---|---|---|
| SH (cm) | CLL (cm) | CLW (cm) | PH (cm) | SD (cm) | HFB (cm) | CD (cm) | |
| Treatments | 9.64** | 1.62** | 0.14** | 892.58** | 0.43** | 120.15** | 2081.91** |
| h2(%) | 95.82 | 94.23 | 88.64 | 98.74 | 99.34 | 94.10 | 98.62 |
| CVg/CVe | 1.69 | 1.43 | 0.99 | 3.13 | 4.32 | 1.41 | 2.99 |
| C.V. (%) | 14.28 | 13.05 | 16.21 | 12.76 | 12.24 | 30.04 | 13.34 |
| F.V. | Characters | ||||||
| LL (cm) | LW (cm) | CLA | CLB | FD (cm) | PL (cm) | NP | |
| Treatments | 11.17** | 1.46** | 528.08** | 72.92** | 0.45** | 0.07** | 0.57** |
| h2(%) | 95.64 | 96.43 | 97.37 | 95.19 | 88.59 | 87.16 | 70.71 |
| CVg/CVe | 1.65 | 1.84 | 2.15 | 1.57 | 0.98 | 0.92 | 0.55 |
| C.V. (%) | 13.12 | 12.83 | 14.77 | 24.04 | 14.07 | 20.28 | 7.50 |
| F.V. | Characters | ||||||
| NS | AL (cm) | FL (cm) | NFP | FW (g) | FL (cm) | WFD (cm) | |
| Treatments | 0.51** | 0.01** | 0.02** | 8057.89** | 66.89** | 8.59** | 2.63** |
| h2(%) | 65.81 | 82.51 | 74.02 | 98.77 | 99.25 | 98.44 | 99.35 |
| CVg/CVe | 0.49 | 0.77 | 0.59 | 3.17 | 4.06 | 2.81 | 4.37 |
| C.V. (%) | 7.67 | 16.87 | 15.78 | 27.25 | 25.85 | 14.11 | 10.11 |
| F.V. | Characters | ||||||
| SFD (cm) | PDL (cm) | PT (cm) | PLL (cm) | NSF | FFM (g) | DMC | |
| Treatments | 0.63** | 0.67** | 0.02** | 3.82** | 5001.63** | 50.61** | 398.56** |
| h2(%) | 97.99 | 87.19 | 96.41 | 97.04 | 96.60 | 99.39 | 95.88 |
| CVg/CVe | 2.47 | 0.92 | 1.83 | 2.02 | 1.89 | 4.51 | 1.70 |
| C.V. (%) | 14.93 | 14.15 | 17.76 | 19.73 | 29.09 | 24.78 | 27.19 |
** Significant at 1% of error probability by the F test.
SH – Seedling Height; CLL – Cotyledons Leaf Length; CLW – Cotyledons Leaf Width; PH – Plant Height; SD – Stem Diameter; HFB – Height of First Branching; CD – Canopy Diameter; LL – Leaf Length; LW – Leaf Width; CLA – Chlorophyll a; CLB – Chlorophyll b; FD – Flower Diameter; PL – Petal Length; NP – Number of Petals; NS – Number of Stamens; AL – Anther Length; FL – Filament Length; NFP – Number of Fruits per Plant; FW – Fruit Weight; FL – Fruit Length; WFD – Widest Fruit Diameter; SFD – Smallest Fruit Diameter; PDL – Pedicel Length; PT – Pericarp Thickness; PLL – Placenta Length; NSF – Number of Seeds per Fruit; FFM – Fresh Fruit Mass; DMC – Dry Matter Content. Cm (centimeter) and g (grams).
Table 2. Analysis of variance summary: mean squares (MS), heritability (h²%), genetic and environmental coefficient of variance ratio (CVg/CVe) and coefficient of variance (CV%) for 28 quantitative variables of ornamental pepper (Capsicum annuum L.) seedlings, plants, flower and fruits. CCA/UFPB.
Heritability values were high, above 70% for all variables except for number of stamens (65.81). The highest heritability values were for plant height (98.74%), stem diameter (99.34%), canopy diameter (98.62%), number of fruit per plant (98.77%), fruit weight (99.25%), fruit length (98.44%), widest fruit diameter (99.35%) and fresh fruit weight (99.39%) (Table 2). The ratio between the genetic coefficient of variance/environmental coefficient of variance (CVg/CVe)was higher than 1 for most traits including seedling height, cotyledons leaf length, plant height, stem diameter, height of first branching, canopy diameter, leaf length, leaf width, chlorophyll a, chlorophyll b, number of fruit per plant, fruit weight, fruit length, widest fruit diameter, smallest fruit diameter, pericarp thickness, placenta length, number of seed per fruit, fresh fruit weight and dry matter content. The characters that presented a ratio lower than one were cotyledons leaf width, flower diameter, petal length, number of petal, number of stamens, anther length, filament length and pedicel length (Table 2).
The coefficient of variance (CV) percentage varied between 7.50% and 30.04% for the number of petals and height of first branching, respectively (Table 2).
The accessions were grouped by Scott-Knott test at 1% of probability (Table 3) into classes that varied from two to eight depending on the analyzed character. The variable with the highest number of distinct groups was widest fruit diameter, forming eight groups, with mean values varying from 0.63 cm for UFPB132 access to 2.49 cm for UFPB001 (Table 3).
| Accessions | Characters | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SH | SLL | CLW | PH | SD | HFB | CD | LL | LW | CLA | CLB | FD | PL | NP | |
| UFPB001 | 3.69d | 2.75b | 1.00a | 16.87e | 0.65b | 5.50c | 24.06f | 6.56 a | 2.09 b | 30.03 b | 8.11 c | 1.84 a | 0.50 c | 5.83 a |
| UFPB002 | 4.70c | 1.77d | 0.71b | 40.50b | 0.25f | 9.82b | 67.25a | 5.79 b | 1.49 d | 20.80 d | 5.23 d | 1.66 b | 0.45 c | 5.37 b |
| UFPB003 | 5.06b | 2.05d | 0.73b | 35.87c | 0. 17g | 2.50d | 59.25b | 4.31 c | 1.19 e | 26.18 c | 6.45 c | 1.85 a | 0.66 a | 5.75 a |
| UFPB004 | 3.06e | 2.11d | 0.56c | 19.00e | 0.59c | 5.66c | 23.42f | 6.82 a | 2.11 b | 28.57 b | 7.56 c | 1.85 a | 0.56 b | 5.66 a |
| UFPB45 | 6.15a | 2.34c | 0.82b | 47.87a | 0.36e | 12.56b | 44.25d | 3.04 d | 1.51 d | 11.40 e | 3.63 d | 1.77 a | 0.54 b | 5.50 a |
| UFPB46 | 4.10d | 2.07d | 0.81b | 40.50b | 0.26f | 11.06b | 67.50a | 4.79 c | 1.88 c | 23.76 c | 7.01 c | 1.70 b | 0.43 c | 5.62 a |
| UFPB77.3 | 2.94e | 2.22c | 0.60c | 32.25c | 0.64b | 15.87a | 35.12e | 5.68 b | 1.87 c | 34.66 a | 13.23 a | 1.28 c | 0.35 d | 5.12 b |
| UFPB099 | 2.87e | 2.06d | 0.75b | 18.44e | 0.74a | 5.81c | 24.00f | 7.15 a | 2.24 b | 29.18 b | 7.72 c | 1.91 a | 0.52 b | 5.87 a |
| UFPB132 | 6.11a | 2.31c | 0.82b | 33.00c | 0.23f | 11.19b | 48.50d | 3.85 d | 1.25 e | 12.72 e | 3.59 d | 1.15 c | 0.40 d | 5.00 b |
| UFPB134 | 5.85 a | 3.50a | 0.76b | 17.56e | 0.52d | 8.19c | 24.37f | 6.13 b | 1.59 d | 36.42 a | 12.94 a | 1.59 b | 0.46 c | 5.33 b |
| UFPB137 | 4.44c | 2.90b | 0.91a | 22.69d | 0.74a | 10.69b | 30.50e | 5.86 b | 1.83 c | 31.94 a | 10.07 b | 1.59 b | 0.42 c | 5.33 b |
| UFPB356 | 3.75d | 2.27c | 0.81b | 23.87d | 0.71a | 14.60a | 26.12f | 5.25 b | 1.72 d | 32.18 a | 10.76 b | 1.51 b | 0.48 c | 5.24 b |
| UFPB390 | 4.25c | 2.75c | 0.85b | 23.31d | 0.65b | 11.44b | 27.37f | 5.42 b | 1.56 d | 28.65 b | 7.52 c | 1.51 b | 0.36 d | 5.16 b |
| UFPB443 | 4.62c | 1.76d | 0.53c | 17.25e | 0.19g | 5.44c | 56.00c | 3.62 d | 1.26 e | 14.65 e | 4.86 d | 1.17 c | 0.34 d | 5.50 a |
| UFPB449 | 5.74a | 2.47c | 0.96c | 19.06e | 0.18g | 7.31c | 32.37e | 6.08 b | 2.82 a | 14.00 e | 5.25 d | 1.71 b | 0.49 c | 5.37 b |
| Calypso | 3.81d | 2.09d | 0.88a | 12.50f | 0.13g | 4.12d | 51.62c | 4.69 c | 1.99 c | 28.77 b | 10.52 b | 1.58 b | 0.65 a | 5.12 b |
SH – Seedling Height; CLL – Cotyledons Leaf Length; CLW – Cotyledons Leaf Width; PH – Plant Height; SD – Stem Diameter; HFB – Height of First Branching; CD – Canopy Diameter; LL – Leaf Length; LW – Leaf Width; CLA – Chlorophyll a; CLB – Chlorophyll b; FD – Flower Diameter; PL – Petal Length; NP – Number of Petals. Means followed by the same letter do not differ statistically among themselves, in the same column, by the Scott-Knott criteria (p=0.01).
Tables 3a. Means of 28 quantitative characters for seedling, plant, flower, and fruit evaluated in 16 accessions of ornamental pepper (Capsicum annuum L.). CCA/UFPB.
| Accessions | Characters | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NS | AL | FL | NFP | FW | FL | WFD | SFD | PDL | PT | PLL | NSF | FFM | DMC | |
| UFPB001 | 5.83a | 0.25b | 0.41b | 12.78f | 8.73a | 3.89b | 2.49ª | 1.12a | 2.14a | 0.23b | 2.67ª | 108.08a | 7.39a | 10.85c |
| UFPB002 | 5.37b | 0.26b | 0.46b | 36.25d | 1.08e | 2.47d | 0.85g | 0.79c | 2.15a | 0.12d | 1.65c | 26.50d | 0.79e | 38.05a |
| UFPB003 | 5.75a | 0.27b | 0.55a | 18.50f | 1.34e | 2.72c | 0.94g | 0.74c | 2.04b | 0.12d | 1.73c | 30.00d | 1.06e | 24.38b |
| UFPB004 | 5.62a | 0.25b | 0.42b | 14.00f | 9.07a | 4.43a | 2.24b | 1.22a | 1.97b | 0.23b | 2.83ª | 75.71b | 7.90a | 9.89c |
| UFPB45 | 5.50a | 0.28b | 0.56a | 17.25f | 1.81d | 2.75c | 1.09f | 0.57e | 2.45a | 0.16c | 1.97b | 19.75d | 1.50d | 13.96c |
| UFPB46 | 5.62a | 0.28b | 0.43b | 16.75f | 2.99c | 2.75c | 1.50e | 0.65d | 2.42a | 0.12d | 2.03b | 48.41d | 2.10c | 13.54c |
| UFPB77.3 | 5.12b | 0.21c | 0.39b | 119.37a | 0.39e | 0.99f | 0.72h | 0.47e | 1.86b | 0.09d | 0.65e | 29.00d | 0.21e | 9.79c |
| UFPB099 | 5.83a | 0.25b | 0.42b | 13.12f | 7.05b | 4.33a | 2.07c | 0.94b | 2.19a | 0.21b | 2.84a | 77.29b | 5.92b | 11.04c |
| UFPB132 | 5.12b | 0.22c | 0.44b | 25.37e | 0.89e | 2.76c | 0.63h | 0.62d | 1.68c | 0.13c | 2.01b | 20.75d | 0.62e | 14.98c |
| UFPB134 | 528b | 0.27b | 0.46b | 38.50d | 1.65d | 2.45d | 1.18f | 0.46e | 2.00b | 0.16c | 1.59c | 58.92c | 1.25d | 15.19c |
| UFPB137 | 5.33b | 0.23c | 0.52a | 42.62d | 1.76d | 2.28d | 1.24f | 0.61d | 2.26a | 0.16c | 1.63c | 61.04c | 1.40d | 12.08c |
| UFPB356 | 5.24b | 0.23c | 0.47b | 54.62c | 0.56e | 0.97f | 0.89g | 0.70c | 1.51c | 0.11d | 0.66e | 25.50d | 0.36e | 15.44c |
| UFPB390 | 5.12b | 0.22c | 0.43b | 102.12b | 0.49e | 1.52e | 0.75h | 0.42e | 2.01b | 0.10d | 1.03d | 33.21d | 0.36e | 11.64c |
| UFPB443 | 5.50a | 0.23c | 0.44b | 33.50d | 0.85e | 1.46e | 0.93g | 0.43e | 1.66c | 0.14c | 1.01d | 29.87d | 0.67e | 11.81c |
| UFPB449 | 5.37b | 0.36a | 0.52a | 10.87f | 3.13c | 3.06c | 1.78d | 1.15a | 2.42a | 0.27a | 1.21d | 36.25d | 2.77c | 13.02c |
| Calypso | 5.12b | 0.27b | 0.49b | 27.62e | 2.09d | 2.67c | 1.41e | 1.14a | 2.41a | 0.13c | 1.75c | 36.20d | 1.59d | 12.84c |
NS – Number of Stamens; AL – Anther Length; FL – Filament Length; NFP – Number of Fruits per Plant; FW – Fruit Weight; FL – Fruit Length; WFD – Widest Fruit Diameter; SFD – Smallest Fruit Diameter; PDL – Pedicel Length; PT – Pericarp Thickness; PLL – Placenta Length; NSF – Number of Seeds per Fruit; FFM – Fresh Fruit Mass; DMC – Dry Matter Content. Means followed by the same letter do not differ statistically among themselves, in the same column, by the Scott-Knott criteria (p=0.01).
Tables 3b. Means of 28 quantitative characters for seedling, plant, flower, and fruit evaluated in 16 accessions of ornamental pepper (Capsicum annuum L.). CCA/UFPB.
Stem diameter was divided into seven classes with the highest mean value for accessions UFPB099 (0.74), UFPB137 (0.74) and UFPB356 (0.71) (Table 3). Plant height, canopy diameter and number of fruits per plant formed six groups each (Table 3). Commercial variety Calypso showed smallest plant height (12.50), followed by accessions UFPB001 (16.87), UFPB443 (17.25), UFPB134 (17.56), UFPB099 (18.44), UFPB004 (19.00) Table 2. Analysis of variance summary: mean squares (MS), heritability (h²%), genetic and environmental coefficient of variance ratio (CVg/CVe) and coefficient of variance (CV%) for 28 quantitative variables of ornamental pepper (Capsicum annuum L.) seedlings, plants, flower and fruits. CCA/UFPB. and UFPB449 (19.06) (Table 3). Accessions UFPB004 (23.42), UFPB099 (24.00), UFPB001 (24.06), UFPB134 (24.37), UFPB356 (26.12) and UFPB390 (27.37) presented lowest mean values for canopy diameter (Table 3).
Accessions UFPB390 (102.12) and UFPB77.3 (119.37) were the ones with the highest number of fruits per plant. As to the fruit length trait, accessions UFPB356 (0.97) and UFPB77.3 (0.99) had the lowest mean values (Table 3). Five groups were formed for seedling height, leaf width, chlorophyll a, smallest fruit diameter, fruit weight, placenta length and fresh fruit mass (Table 3). Accessions UFPB449 (5.74), UFPB134 (5.85), UFPB132 (6.11) and UFPB45 (6.15) presented the tallest seedlings (Table 3). Leaf width was smaller in accessions UFPB003, UFPB132 and UFPB443 with mean values of 1.19, 1.25 and 1.26, respectively (Table 3). Accessions UFPB137 (31.94), UFPB356 (32.18), UFPB77.3 (34.66) and UFPB134 (36.42) presented the highest values of chlorophyll a (Table 3).
The treatments with the lowest mean values for smallest fruit diameter were UFPB390 (0.42), UFPB443 (0.43), UFPB134 (0.46), UFPB77.3 (0.47) and UFPB45 (0.57) (Table 3).The accessions with the lowest values for fruit weight were UFPB77.3 (0.39), UFPB390 (0.49), UFPB356 (0.56), UFPB443 (0.85), UFPB132 (0.89), UFPB002 (1.08) UFPB003 (1.34) (Table 3).Accessions UFPB001, UFPB004 and UFPB099 had the highest means for placenta length (2.67, 2.83 and 2.84, respectively), and UFPB77.3 had the lowest mean value (0.65) (Table 3).
Accessions with the highest values for fresh fruit mass were UFPB001 (7.39) and UFPB004 (7.90) (Table 3).The following characters were grouped into four classes: cotyledons leaf length, height of first branching, leaf length, chlorophyll b, petal length, pericarp thickness and number of seeds per fruit. Accession UFPB134 presented the lowest mean value of 3.50 for cotyledons leaf length.Accession UFPB003 (2.50) and Calypso (4.12) showed the lowest mean values for height of first branching (Table 3). As to leaf length, accessions UFPB45, UFPB443 and UFPB132 had the lowest mean values of 3.04, 3.62 and 3.85, respectively.
Accessions UFPB134 (12.94) and UFPB77.3 (13.23) had the highest values of chlorophyll b. For petal length, the Calypso variety and the UFPB003 accession presented the longest petals, with mean values of 0.65 and 0.66, respectively. As to pericarp thickness, accession UFPB449 (0.27) presented the highest mean value for this characteristic (Table 3). Accession UFPB001 (108.08) had the highest mean value for the number of seed per fruit trait.Cotyledons leaf width, flower diameter, anther length, pedicel length and dry matter content formed three groups (Table 3). Accessions UFPB137 (0.91), UFPB001 (1.00) and Calypso (0.88) had the highest mean values for the characteristic cotyledons leaf width (Table 3).Accessions UFPB001 (1.84), UFPB004 (1.85), UFPB099 (1.91), UFPB45 (1.77) and UFPB003 (1.85) had the highest mean values for flower diameter.
Regarding anther length, accession UFPB449 (0.36) was the one with the highest mean value.The highest means for pedicel length were from accessions UFPB001 (2.14 cm), UFPB002 (2.15 cm), UFPB099 (2.19 cm), UFPB137 (2.26 cm), Calypso (2.41 cm), UFPB46 (2.42 cm) and UFPB45 (2.45 cm).
Accession UFPB002 (38.05) showed the highest mean value for dry matter content.The characteristics of number of petals, number of stamens and filament length, formed only two groups, presenting lower variability among the evaluated accessions (Table 3). For all three traits, accessions UFPB001, UFPB002, UFPB004, UFPB46, UFPB77.3, UFPB099, UFPB132, UFPB134, UFPB356, UFPB390, UFPB443 and Calypso had the highest mean values (Table 3).
After the analysis of variance, through the Tocher method, the accessions were divided into five groups (Table 4) Group I is composed of three accessions (18.75%), UFPB001, UFPB004 and UFPB099. This group presented similar means for port characters (plant height, height of first branching, canopy diameter, leaf length and width), chlorophyll (a and b), flower characters (flower diameter, number of petals, number of stamens, anther length and filament length) and fruit characters (number of fruits per plant, pericarp thickness, placenta length and dry matter content).
| Groups | Accessions |
|---|---|
| 1 | UFPB001, UFPB004 e UFPB099 |
| 2 | UFPB77.3, UFPB134, UFPB137, UFPB390 e UFPB356 |
| 3 | UFPB002, UFPB003, UFPB45 e UFPB46 |
| 4 | UFPB132 e UFPB443 |
| 5 | UFPB449 e Calypso |
Table 4. Clustering of 16 accessions, based on 28 quantitive traits of ornamental pepper (Capsicum annuum L.) by the Tocher method. CCA /UFPB. Areia, 2016.
Group II consisted of five accessions, UFPB77.3, UFPB134, UFPB137, UFPB356 and UFPB390, gathering 31.25% of the total evaluated. These accessions presented similar mean data for leaf length, number of petals and dry matter content. Group III comprised four accessions, UFPB002, UFPB003, UFPB45 and UFPB46, representing 25% of the individuals analyzed. In this group, similar mean values were found for cotyledons leaf width, anther length and number of seeds per fruit. Group IV consisted of two accessions, UFPB132 and UFPB443 (12.5%), which presented similar mean values for leaf length and width, chlorophyll (a and b), flower diameter, petal length, anther length, filament length, fruit weight, fruit length, pericarp thickness, number of seeds per fruit, fresh fruit mass and dry matter content.
Group V is composed of two accessions, UFPB449 and the Calypso variety (12.5%), which showed similarities in the following traits: stem diameter, flower diameter, number of petals, number of stamens, fruit length, smallest fruit, pedicel length, number of seeds per fruit and dry matter content.
Through the Singh method (1981), it was determined that eight of the 28 characteristics contributed with 76.85% of the genetic divergence, while 20 contributed with only 23.15% (Table 5). Among the studied characters, the ones that most contributed to the genetic divergence between accessions were: fresh fruit mass (24.38%), stem diameter (14.85%), widest fruit diameter (11.68%), fruit weight (11.29%), plant height (6.67%), canopy diameter (5.24%), chlorophyll a (4.93%) and number of fruits per plant (4.68%) (Table 5).
| Variables | Relative contribution | |
|---|---|---|
| S. j | Value in % | |
| Seedling height | 1039.69 | 1.86 |
| Cotyledons leaf length | 681.29 | 1.22 |
| Cotyledons leaf width | 324.68 | 0.58 |
| Plant height | 3718.68 | 6.67 |
| Stem diameter | 8279.52 | 14.85 |
| Height of first branching | 764.78 | 1.37 |
| Canopy diameter | 2920.24 | 5.24 |
| Leaf length | 302.86 | 0.54 |
| Leaf width | 929.71 | 1.67 |
| Chlorophyll a | 2752.23 | 4.93 |
| Chlorophyll b | 183.68 | 0.33 |
| Flower diameter | 62.41 | 0.11 |
| Petal length | 221.54 | 0.39 |
| Number of petals | 227.72 | 0.41 |
| Number of stamens | 191.17 | 0.34 |
| Anther length | 158.07 | 0.28 |
| Filament length | 228.21 | 0.41 |
| Number of fruits per plant | 2609.45 | 4.68 |
| Fruit weight | 6295.44 | 11.29 |
| Fruit length | 598.80 | 1.08 |
| Widest fruit diameter | 6514.96 | 11.68 |
| Smallest fruit diameter | 133.13 | 0.24 |
| Pedicel length | 246.64 | 0.44 |
| Pericarp thickness | 516.51 | 0.93 |
| Placenta length | 151.83 | 0.27 |
| Number of seeds per fruit | 1126.55 | 2.02 |
| Fresh fruit mass | 13596.11 | 24.38 |
| Dry matter content | 993.48 | 1.78 |
Table 5. Estimates of the realtive contribution of each variable (S.j) to the genetic diversion among Capsicum annuum L. accessions, based on the total D² Mahalanobis distance for 28 morphoagronomic variables of ornamental pepper seedling, plant, flower and fruit. CCA/UFPB.
The variables that least contributed to the divergence were cotyledonous leaf width, leaf length, chlorophyll b, flower diameter, petal length, number of stamens, anther length, filament length, smallest fruit diameter, pedicel length, pericarp thickness and placenta length (Table 5).
The first two canonical variances of the canonical variables represent 70.4% of the total variation (Table 6).
| Canonic Variables | Eigenvalues | Eigenvalues % | Accumulated % |
|---|---|---|---|
| CV1 | 75.08 | 46.12 | 46.12 |
| CV2 | 39.53 | 24.28 | 70.41 |
| CV3 | 13.82 | 8.49 | 78.90 |
| CV4 | 9.16 | 5.62 | 84.53 |
| CV5 | 7.07 | 4.34 | 88.88 |
| CV6 | 4.81 | 2.96 | 91.84 |
| CV7 | 3.74 | 2.29 | 94.14 |
| CV8 | 2.99 | 1.84 | 95.98 |
| CV9 | 2.28 | 1.40 | 97.38 |
| CV10 | 1.40 | 0.86 | 98.24 |
| CV11 | 1.17 | 0.72 | 98.96 |
| CV12 | 0.83 | 0.51 | 99.48 |
| CV13 | 0.38 | 0.23 | 99.72 |
| CV14 | 0.30 | 0.18 | 99.90 |
| CV15 | 0.15 | 0.09 | 100.00 |
| CV16 | 0.00 | 0.00 | 100.00 |
| CV17 | 0.00 | 0.00 | 100.00 |
| CV18 | 0.00 | 0.00 | 100.00 |
| CV19 | 0.00 | 0.00 | 100.00 |
| CV20 | 0.00 | 0.00 | 100.00 |
| CV21 | 0.00 | 0.00 | 100.00 |
| CV22 | 0.00 | 0.00 | 100.00 |
| CV23 | 0.00 | 0.00 | 100.00 |
| CV24 | 0.00 | 0.00 | 100.00 |
| CV25 | 0.00 | 0.00 | 100.00 |
| CV26 | 0.00 | 0.00 | 100.00 |
| CV27 | 0.00 | 0.00 | 100.00 |
| CV28 | 0.00 | 0.00 | 100.00 |
Table 6. Estimates of variance (eigenvalues) associated to canonic variables, related to 28 morphoagronomic characters of ornamental pepper (Capsicum annuum L.) seedling, plant, flower and fruit. CCA/UFPB.
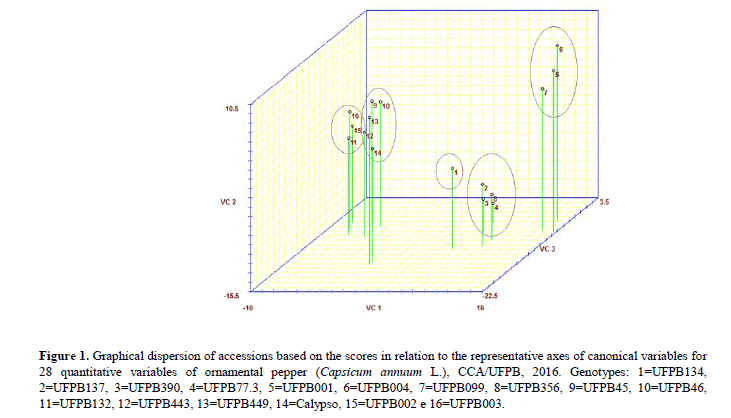
The graphic dispersion of the accessions, using the canonical variables’ scores, formed five groups (Figure 1). The first group gathered the most accessions: UFPB45, UFPB46, UFPB443, UFPB449 and the cultivar Calypso. The second group consisted of four accessions: UFPB77.3, UFPB137, UFPB390 and UFPB356.Accessions UFPB001, UFPB004 and UFPB099 made up the third group.The fourth group comprised the accessions UFPB002, UFPB003 and UFPB132.Accession UFPB134 formed the fifth group.
Figure 1: Graphical dispersion of accessions based on the scores in relation to the representative axes of canonical variables for 28 quantitative variables of ornamental pepper (Capsicum annuum L.), CCA/UFPB, 2016. Genotypes: 1=UFPB134, 2=UFPB137, 3=UFPB390, 4=UFPB77.3, 5=UFPB001, 6=UFPB004, 7=UFPB099, 8=UFPB356, 9=UFPB45, 10=UFPB46, 11=UFPB132, 12=UFPB443, 13=UFPB449, 14=Calypso, 15=UFPB002 e 16=UFPB003.
Discussion
The significant difference observed among treatments for all evaluated characters confirms the genetic diversity between accessions. The results show that the accessions behaved differently for each evaluated trait, which allows gain in the selection process. Leite et al. (2016) and Martinez et al. (2017) found genetic variability among accessions of C. baccatum to quantitative traits related to plant, flower and fruits. Neitzke et al. (2010) reported divergence among genotypes for plant and fruit traits in pepper with ornamental potential. All these results demonstrate the existence of variability between the evaluated genotypes and, consequently, the possibility of obtaining genetic gains in breeding programs (Elias et al., 2007).
The importance of genetic diversity for breeding lies in the fact that crosses involving genetically divergent parents are the most appropriate to produce a high heterotic effect, as well as greater genetic variability in segregating generations (Rao et al., 1981; Bahia et al., 2008).
High heritability values found among the accessions for the evaluated characteristics indicate that the differences found between them are due to genetic variation rather than to environmental variation. The genetic value is then transmitted to the next generation, and the higher a trait’s heritability is the more reliable is the selection. These results were confirmed by Silva et al. (2016), in a study on the correlation network analysis between phenotypic and genotypic characters of pepper plants, which obtained high heritability values, that is to say, the phenotypic variability in these genotypes is mainly determined by genotypic variability.
High heritability values show a high genetic control and favor the selection of traits under study (Rosado et al., 2009). Medeiros et al. (2015) and Pessoa et al. (2015a) also verified high heritability values (upwards of 70%) for characters related to seed germination in pepper plants. Fortunato et al. (2015) and Pessoa et al. (2015b) also found high values of heritability for port characters, demonstrating that most of the observed phenotypic variation is of genetic origin. Rêgo et al. (2011) found heritability values higher than 80% for characters related to pepper fruits as well. These results indicate that different pepper characters present high heritability values and that selection can be focused on these phenotypes, which is favorable for genetic breeding.
Heritability values vary according to the characteristic, with populations formed from divergent parents presenting greater variability (Borém and Miranda, 2013). Heritability is one of the most important genetic parameters, as it quantifies the fraction of phenotypic variation that is inheritable and can be explored in selection (Rosado et al., 2012; Moraes et al., 2015). Thus, it is recommended to select accessions that present characteristics with high heritability values.
The values found for the CVg/CVe ratio were higher than 1 for most characteristics (seedling height, cotyledons leaf length, plant height, stem diameter, height of first branching, canopy diameter, leaf length and width, chlorophyll a and b, number of fruits per plant, fruit weight, fruit length, widest and smallest fruit diameter, pericarp thickness, placenta length, number of seeds per fruit, fresh fruit mass and dry matter content), which evidences the existence of genetic variability and reinforces the indicative that much of the variation observed is genetic in nature. Similar results were found by Rêgo et al. (2010) and Nascimento et al. (2012) when working with port and flower traits in Capsicum, these authors indicated characters with greater genetic variation in order to obtain favorable gains with the selection.
When the values of the genetic coefficient of variance/environmental coefficient of variance (CVg/CVe) are greater than 1, it is an indication that the chances of genetic gain will be high for the evaluated characteristics (Cruz et al., 2012; Leite et al., 2015). In this work, it is possible to predict the possibility of gain in the selection of ornamental pepper accessions in breeding programs, due to high estimates of heritability and genetic variance obtained for most of the evaluated traits for seedlings, plant, flower and fruits.
The variables (cotyledons leaf width, flower diameter, flower length, number of petals, number of stamens, anther length, filament length and pedicel length) that presented values lower than 1 were not favorable for selection, since the genetic gain will be low. They can be used in indirect selection (Cruz et al., 2012) or selection in advanced generations (Nascimento et al., 2012), where it is possible to obtain long-term gain based on these characters. A greater proportion of environmental variation is evidenced in these traits, which makes the situation less favorable for breeding.
The coefficient of variation values found for the traits though low (ranging from 7.50% to 30.04%) are considered satisfactory. Similar results were found by Medeiros et al. (2014) in a study with fruit characters in Capsicum baccatum which reported good experimental precision of data, with a variation of 6% to 24.92%, assuring the validity of the inferred conclusions. CV values lower than 30% are considered low, since the coefficient of variation gives an idea of the experimental precision (Gomes and Garcia, 2002). However, research that is more recent has shown that CV classification must consider the species, the evaluated characteristic, the number of repetitions, the experimental design, among other important aspects (Silva et al., 2011).
The difference found by the mean test (Scott-Knott) between the genotypes confirms the existence of variability among them. This variability is fundamental in genetic breeding programs in order to identify superior plants in segregating progeny (Neto et al., 2010). Rêgo et al. (2010a) found results similar to these in a study about the phenotypic characterization of ornamental pepper plants, detecting through the Scott-Knott test the variability and potential of plants for ornamental use.
The variability identified among the accessions is important in the identification of pepper plants with ornamental potential. Accession UFPB134 is recommended when it comes to seedling traits since it presented high performance for seedling height and cotyledons leaf length, forming vigorous seedlings suitable for transplanting, which in turn allows greater success in establishing the plant and maximizes its growth by decreasing the time of transplantation to the field, according to Pastorinil et al. (2016).
The values found for plant characters evidenced the ornamental potential of some accessions such as UFPB001, UFPB004 UFPB099 and UFPB134, which performed well in plant height and canopy diameter and, therefore, are recommended for selection ir order to develop hybrids or lines of small port pepper plants. In addition to plant height and canopy diameter, height of first branching is also an important trait in the determination of plant port; accession UFPB003 can be selected for these characters. The afore mentioned accessions presented values for plant height and height of first branching similar to the values found for Calypso, which is a commercial cultivar variety of ornamental pepper and presents ideal characteristics for cultivation in vases and is used as indoor decoration.
Ideal height in ornamental pepper can be a difficult parameter to define since it may vary according to the taste of the consumer (Lima et al., 2013). Smaller plants are indicated for cultivation in small vases while larger plants can be grown in larger pots or as outdoor crops. It is recommended that accessions selected for ornamental use have canopy diameter and plant height 1.5 to 2 times larger than the pot it is planted in (Barbosa et al., 2003; Barroso et al., 2012). In this respect, accessions UFPB001, UFPB004, UFPB099 and UFPB134 are indicated for selection, in order to obtain smaller plants that can be grown in small pots and used as interior decoration.
Plants with the highest mean values for stem diameter, such as accessions UFPB 099, UFPB 137 and UFPB356, are recommended for selection. Nascimento et al. (2011) and Neto et al. (2014) when working with ornamental pepper found results similar to this paper’s, indicating for selection genotypes that presented the highest mean values for this trait. This character is important in the selection of genotypes since the stem diameter must be large enough to support the weight of the plant and the fruits (Ferreira et al., 2015).
The variation observed in leaf length and leaf width means allowed us to indicate accession UFPB132 for selection because it presented the smallest leaves in both length and width. According to Barroso et al. (2012), small leaves are of interest for ornamental peppers, in order to maintain harmony with the small sized plant.In addition to the plant characters, the levels of chlorophyll a and chlorophyll b also differed among the genotypes, which indicates influence from the treatments on the pigment production. Accessions UFPB77.3 and UFPB134 are recommended for selection since they presented the highest levels of chlorophyll a and b. Pessoa et al. (2015b) also reported variability for chlorophyll a and b contents in ornamental pepper population, indicating for selection the accessions that showed the highest levels. Chlorophyll content varies greatly among species and among genotypes of the same species (LEE et al., 1988). Chlorophyll is present in all green vegetables and is one of the factors related to the photosynthetic efficiency of plants and, consequently, to plant growth (Engel and Poggiani, 1991). Hence, the accessions that presented the highest levels of chlorophyll (UFPB77.3 and UFPB134) have higher photosynthetic efficiency.
Variability among accessions was also demonstrated among flower characters that formed three or four phenotypic groups. This variability makes it possible to obtain genetic gains in breeding programs (Santos et al., 2011). The selection of genotypes with large flowers is important not only in the ornamental aspect (Santos et al., 2013). The analysis of flower traits is fundamental in all phases of genetic breeding given the need to carry out segregating generations, as well as to produce hybrid and genetic seeds (Rêgo et al., 2012). Taking this into consideration, accessions UFPB001, UFPB003, UFPB004, UFPB45 and UFPB099 are indicated for the selection, because they present larger flowers; also accession UFPB003 and the cultivar Calypso, because they presented the largest petals.
Neto et al. (2014) and Fortunato et al. (2015) found similar results for variability in flower characters in pepper. These results indicate that there are different sized flowers among the genotypes and the one with the largest flowers can be selected. Santos et al. (2013) reported that plants with large flowers have potential for use in ornamental pepper breeding programs because they provide beauty to the plant and are striking and attractive to consumers.
Accession UFPB449 is recommended for selection for anther length since it has the highest value for this characteristic. The results of this research differ from those found by Vasconcelos et al. (2012), which obtained homogeneous values between the accessions of pepper for anther length, evidencing that there were no significant differences for this trait among the genotypes. The importance of anther length as a character lays in the fact that larger anthers make it easier for breeders to emasculate flowers for crossing.
The flower characteristics that formed two groups (number of petals, number of stamens and filament length) indicate little variability among accessions. Some plants showed variation in the number of petals and number of stamens, ranging from five to seven petals and/or stamens. Nascimento et al. (2012) found results similar to this one for filament length, with the formation of two groups for this trait, showing little variability. That evidences low genetic variability for this character, and therefore, little expectation of gain with the selection.
Differences observed for fruit characters indicate that the analyzed accessions present genetic variability and are not the same for these traits. Other than the port and flower characteristics, fruits are one of the main attractions in ornamental pepper (Silva et al., 2015). The fruits’ different forms, sizes and colors make the plants more attractive to consumers (Carvalho et al., 2006).
Accessions UFPB 77.3 and UFPB390 are indicated for selection for the characters number of fruits per plant, fruit weight, fruit length, widest and smallest fruit diameter, because they present the highest number of fruits per plant and small fruits. Generally, fruits with reduced diameters are small and less heavy. Accessions with small fruits in large quantities are recommended for use in ornamental pepper breeding, since they stand out in the foliage (Bosland, 1993; Sudré et al., 2005; Büttow et al. 2016).
Accessions UFPB001, UFPB002, UFPB45, UFPB46, UFPB099, UFPB137 and UFPB449, as well as the Calypso cultivar are indicated for selection in pedicel length. Pedicel length is an interesting characteristic in ornamental peppers because fruits with greater length are more prominent in relation to the leaves, which is attention-grabbing in potted plants (Melo et al., 2014) and also facilitates fruit harvesting. Büttow et al. (2010) reported similar observations, studying genetic diversity between accessions of chili peppers and peppers, suggesting the selection of plants whose fruits stood out in the foliage, with long pedicels.
Another important trait to be considered in the selection of accessions is pericarp thickness. Access UFPB449 is indicated for selection because it has a thicker pericarp. This character is directly correlated with the production (Rêgo et al., 2011) and influences increase in fruit firmness. It is an important aspect in fruit quality, since firm fruits tolerate damages better, allowing commercialization for longer periods of time (Ferrão et al., 2011).
The highest mean values for placenta length, number of seeds per fruit and fresh fruit matter indicate accessions UFPB001, UFPB004 and UFPB099 for selection. These characteristics varied according to fruit size.The sorting of genotypes into five groups by the Tocher optimization method indicates the existence of variability for the characteristics evaluated. Accessions that are part of the same group are more similar but are not recommended for use in hybrid breeding programs, so that the variability, which is indispensable in any breeding program, is not restricted and gains in selection made unviable (Correa and Gonçalves, 2012).
Group I accessions (UFPB001, UFPB004 and UFPB099) are promising for ornamental use in vases because they are small plants with large flowers. In ornamental pepper it is recommended to select plants with small port (Finger et al., 2012), large flowers and small fruits. Accessions in group II (UFPB77.3, UFPB134, UFPB137, UFPB390 and UFPB356) had better performances for fruit characters, such as higher quantity of fruits per plants and small fruits, aspects of interest for ornamental pepper breeding focused on cultivation in pots and use in interior decorating. In this case, we suggest that this group’s genotypes are crossed with genotypes from group I in order to incorporate these aesthetic values to the characteristics of that group.
Group III accessions (UFPB002, UFPB003, UFPB45 and UFPB46) are indicated for selection of high plants. These characteristics are not indicated for ornamental purposes, because tall plants are undesirable for cultivation in small pots, but they are recommended for outdoor crops. Neitzke et al. (2010) report that high pepper plants can be grown in functional gardens, such as spices, medicinal and aromatic gardens. The accessions that constituted group IV (UFPB132 and UFPB443) presented characteristics of interest for ornamental pepper breeding, such as small leaves and fruits. Barroso et al. (2012) reported that small leaves are preferred for ornamental pepper because they maintain harmony with the plant’s port. Small fruits, on the other hand, indicate a greater possibility of obtaining erect fruits, which are more prominent among the foliage. These fruits are ideal for cultivation in small pots, due to the small size of the plants (Silva et al., 2015).
Group V accessions (UFPB449 and Calypso) presented characteristics of interest for ornamental purposes, such as small port plants and large pedicels. These traits are important for potted crops because large pedicels can highlight flowers and fruits among the foliage (Melo et al., 2014). Besides the afore mentioned characters, the cultivar Calypso also stands out for showing larger petals and smaller fruits when compared to the genotypes UFPB001, UFPB004 and UFPB099. This cultivar is very popular among ornamental pepper plants, and it is cultivated in Brazil and other countries (Finger et al., 2015).
The directed crossing between genotypes belonging to contrasting groups may lead to creation of segregating families with high productive potential and an increase in the probability of recovering superior genotypes in segregating generations (Stähelin et al., 2011). There is a growing demand for new ornamental pepper cultivars that have small port, fruit that stands out among the leaves, and post-production quality (Rêgo and Rêgo, 2016). Other works with Capsicum have shown variability among genotypes through Tocher grouping. Rêgo et al. (2010) while working on the diversity among six ornamental pepper genotypes formed 3 groups consisting of different accessions. Faria et al. (2012) in a work about clustering methods applied in a study of genetic divergence of pepper plants reported the formation of four groups for 49 evaluated genotypes. Neto et al. (2014) working with ornamental pepper population, reported the formation of eight groups in a study of 54 genotypes. All these researches demonstrated the existence of variability among the genotypes. Bianchi et al. (2016) in a study with pepper, report that morphoagronomic characterization is efficient in estimating genetic diversity among genotypes, since it illustrates divergence, which is an important tool for breeding.
A group of genotypes is divided into subgroups by the Tocher optimization method, based on the criterion that the average dissimilarity measurements within each group should be smaller than the average distances between any groups (Vasconcelos et al., 2007).
The characters (fresh fruit mass, stem diameter, largest fruit diameter, fruit weight, plant height, canopy diameter, chlorophyll a and number of fruits per plant) contributed the most to genetic divergence, according to the results from Singh’s method (1981), indicating that these traits are more efficient in explaining the dissimilarity among the 16 evaluated genotypes. Rêgo et al. (2011) in a study with Capsicum baccatum, reported that widest fruit diameter was one of the characteristics that had greater degree of contribution to divergence among genotypes as well. This find indicates that the mentioned traits should be prioritized in studies of divergence among ornamental pepper accessions.
According to the results found in this study, the characters for cotyledonous leaf length, leaf length, chlorophyll b, flower diameter, petal length, number of stamens, anther length, filament length, smallest fruit diameter, pedicel length, pericarp thickness and placenta length, can be discarded in future researches, since they did not contribute to the differentiation of genotypes in a diversity study. To discard variables, we try to identify the traits whose variance is zero or very close to zero (Cruz et al., 2011).
The results from the canonical variables were satisfactory with total variations above 70% obtained in the first three canonical variables, which allowed the analysis of accessions groups through graphic dispersion, therefore this method can be used in future studies. Bento et al. (2007) observed similar results in a study on phenotypic variability in peppers, where they found values in which the first three canonical variables explained more than 70% of the data variation. Ferrão et al. (2011) also used canonical variables to complement the grouping analysis in a research on genetic divergence among pepper genotypes with formation of dispersion graphs. This shows that the employed traits discriminated the analyzed genotypes satisfactorily (Carmona et al., 2015).
The amount of groups formed in the accessions dispersion graph were consistent with the amounts formed through Tocher clustering. However, the composition of each group differed and only group 5 (UFPB001, UFPB004 and UFPB099) was the same in both methods. The analysis of the clusters established by the Tocher method and canonical variable allows the identification of the genotypes that can result in variability in segregating generations.
Conclusion
There is genetic divergence among accessions, thus enhancing their use in breeding programs. Accessions UFPB001, UFPB004, UFPB45, UFPB77.3, UFPB099, UFPB134, UFPB390 and Calypso are indicated as potential ornamental pepper ideal, with vigorou seedlings, small port, large flowers and small fruits. Ornamental pepper accessions with longer anthers are indicated for selection because they make it easier for breeders to emasculate flowers for crossing.
About the Authors
Corresponding Author
Angela Maria dos Santos Pessoa
Universidade Federal da Paraíba, Laboratório de Biotecnologia Vegetal, Rodovia PB 079, CEP: 58397-000 Areia, PB, Brazil
- Email:
- angelapessoapb@gmail.com
References
- Bahia HF, Silva AS, Fernandez LG, Ledo AS, Moreira RFC (2008). Divergência genética entre cinco cultivares de mamoneira. Pesquisa Agropecuária Brasileira. 43: 357-362. https://doi.org/10.1590/s0100-204x2008000300010
- Barbosa JG (2002). Crisântemo: produção de mudas, cultivo para corte de flor, cultivo em vaso, cultivo hidropônico. Ed. Aprenda Fácil, Viçosa. 232.
- Barroso PA, Rêgo ER, Rêgo MM, Nascimento KS, et al. (2012). Analysis of Segregating Generation for Components of Seedling and Plant Height of Pepper (Capsicum annuum L.) for Medicinal and Ornamental Purposes. Acta Horticulturae. 953: 269-275. https://doi.org/10.17660/actahortic.2012.953.37
- Bento CS, Sudre CP, Rodrigues R, Riva EM, et al. (2007). Descritores qualitativos e multicategóricos na estimativa da variabilidade fenotípica entre acessos de pimentas. Scientia Agrária. 8: 149-156. https://doi.org/10.5380/rsa.v8i2.8379
- Bianchi PA, Dutra IP, Moulin MM, Santos JO, et al. (2016). Morphological characterization and analysis of genetic variability among pepper accessions. Ciência Rural. 46: 1151-1157. https://doi.org/10.1590/0103-8478cr20150825
- Bosland PW (1993). Breeding for quality Capsicum. Capsicum and Eggplant Newsletter. 12: 25-31.
- Borém A, Miranda GV (2013). Melhoramento de plantas. 6. ed. Viçosa: UFV.
- Büttow MV, Barbieri RL, Neitzke RS, Heiden G, et al. (2010). Diversidade genética entre acessos de pimentas e pimentões da Embrapa Clima Temperado. Ciência Rural. 40: 1264-1269. https://doi.org/10.1590/s0103-84782010000600004
- Carmona PAO, Peixoto JR, Amaro GB, Mendonça MA (2015). Divergência genética entre acessos de batata-doce utilizando descritores morfoagronômicos das raízes. Horticultura Brasileira. 33: 241-250. https://doi.org/10.1590/s0102-053620150000200017
- Carvalho SIC, Bianchetti LB, Ribeiro CSC, Lopes CA (2006). Pimentas do gênero Capsicum no Brasil. Brasília: Embrapa Hortaliças. 27p.
- Carvalho SIC, Bianchetti LB, Bustamante PG, Silva DB (2003). Catálogo de Germoplasma de pimentas e pimentões (Capsicum spp.) da Embrapa Hortaliças. Brasília: Embrapa Hortaliças. 49p.
- Correa AM, Gonçalves M.C (2012). Divergência genética em genótipos de feijão comum cultivados em Mato Grosso do Sul. Revista Ceres. 59: 206-212. https://doi.org/10.1590/s0034-737x2012000200009
- Costa LV, Bentes JLS, Lopes MTG, Alves SEM (2015). Caracterização de acessos de pimentas do Amazonas. Horticultura Brasileira. 33: 290-298. https://doi.org/10.1590/s0102-053620150000300003
- Cruz, CD (2006). Programa genes (versão Windows): aplicativo computacional em genética e estatística. Editora UFV (Universidade Federal de Viçosa), Viçosa.
- Cruz CD, Ferreira FM, Pessoni LA (2011). Biometria aplicada ao estudo da diversidade genética. Visconde do Rio Branco: Suprema. 620.
- Cruz CD, Regazzi AJ, Carneiro PCS (2012). Modelos biométricos aplicados ao melhoramento genético. 4.ed. Viçosa: UFV. 514p.
- Elias HT, Vidigal MCG, Gonela A, Vogt GA (2007). Variabilidade genética em germoplasma tradicional de feijão-preto em Santa Catarina. Pesquisa Agropecuária Brasileira. 42. 1443-1449. https://doi.org/10.1590/s0100-204x2007001000011
- Engel VL, Poggiani F (1991). Estudo da concentração de clorofila nas folhas e seu espectro de absorção de luz em função do sombreamento em mudas de quatro espécies florestais nativas. Revista Brasileira de Fisiologia Vegetal 3: 39-45.
- Faria PN, Cecon PR, Silva AR, Finger FL, et al. (2012). Métodos de agrupamento em estudo de divergência genética de pimentas. Horticultura Brasileira. 30: 428-432 https://doi.org/10.1590/s0102-05362012000300012
- Ferrão LFV, Cecon PR, Finger FL, Silva FF (2011). Divergência genética entre genótipos de pimenta com base em caracteres morfo-agrônomicos. Horticultura Brasileira. 29: 354-358. https://doi.org/10.1590/s0102-05362011000300016
- Ferreira KTC, Rêgo ER, Rêgo MM, Fortunato FLG (2015). Combining Ability for Morpho-Agronomic Traits in Ornamental Pepper. Acta Horticulturae. 1087. 187-194. https://doi.org/10.17660/actahortic.2015.1087.22
- Finger FL, Rêgo ER, Segatto FB, Nascimento NFF (2012). Produção e potencial de mercado para pimenta ornamental. Informe Agropecuário. 33: 14-20.
- Finger FL, Silva TP, Segatto FB, Barbosa JG (2015). Inhibition of ethylene response by 1-methylcyclopropene in potted ornamental pepper. Ciência Rural. 45: 964-969. https://doi.org/10.1590/0103-8478cr20131386
- Fortunato FLG, Rêgo ER, Santos CAP, Carvalho MG (2015). Heritability and Genetic Parameters for Size-Related Traits in Ornamental Pepper (Capsicum annuum L.). Acta Horticulturae.1087: 201-206. https://doi.org/10.17660/actahortic.2015.1087.24
- Gomes FP, Garcia CH (2002). Estatística aplicada a experimentos agronômicos e florestais: exposição com exemplos e orientações para uso de aplicativos. Piracicaba: FEALQ. 309p.
- IPGRI (1995). Descriptores para Capsicum (Capsicum spp). Roma: IPGRI, 51p.
- Lee DW (1988). Simulating forest shade to study the development ecology of tropical plants: juvenile growth in three vines in India. Journal of Tropical Ecology. 4: 281-292. https://doi.org/10.1017/s0266467400002844
- Leite PSS, Rodriguês R, Silva RNO, Pimenta S, et al. (2016). Molecular and agronomic analysis of intraspecific variability in Capsicum baccatum var. pendulum accessions. Genetics and Molecular Research. 15: 1-16. https://doi.org/10.4238/gmr.15048482
- Leite WS, Pavan BE, Matos Filho CHA, Feitosa FS, et al. (2015). Estimativas de parâmetros genéticos e correlações entre caracteres agronômicos em genótipos de soja. Pesquisas Agrárias e Ambientais. 3: 241- 245. https://doi.org/10.14583/2318-7670.v03n04a03
- Lima IB, Santos AB, Fonseca JJS, Takane RJ, et al. (2013). Pimenteira ornamental submetida a tratamentos com daminozide em vasos com fibra de côco ou areia. Semina: Ciências Agrárias. 34: 3597- 3610. https://doi.org/10.5433/1679-0359.2013v34n6supl1p3597
- Martinez ALA, Araújo JSP, Ragassi CF, Buso GSC (2017). Variability among Capsicum baccatum accessions from Goiás, Brazil, assessed by morphological traits and molecular markers. 16: 1-13. https://doi.org/10.4238/gmr16039074
- Medeiros AM, Rodrigues R, Gonçalves LSA, Sudré CP, et al. (2014). Gene effect and heterosis in Capsicum baccatum var. pendulum. Ciência Rural. 44: 1031-1036.
- Medeiros GDA, Rêgo ER, Barroso PA, Ferreira KTC, et al. (2015). Heritability of Traits Related to Germination and Morphogenesis In Vitro in Ornamental Peppers. Acta Horticuture. 1087: 403-408. https://doi.org/10.17660/actahortic.2015.1087.54
- Melo LF, Gomes RLF, Silva VB, Monteiro ER, et al. (2014). Potencial ornamental de acessos de pimenta. Ciência Rural. 44: 2010-2015. https://doi.org/10.1590/0103-8478cr20131306
- Moraes CB, Carvalho EV, Zimback L, Luz OSL, et al. (2015). Variabilidade genética em progênies de meios-irmãos de eucaliptos para tolerância ao frio. Revista Árvore. 39: 1047-1054. https://doi.org/10.1590/0100-67622015000600007
- Nascimento NFF, Nascimento MF, Rêgo ER, Rêgo MM (2011). Caracterização morfoagronomica em híbridos interespecíficos de pimenteiras ornamentais. Horticultura Brasileira. 29: 2932-2939.
- Nascimento NFF, Rêgo ER, Nascimento MF, Finger FL, et al. (2012). Heritability and variability of morphological traits in a segregating generation of ornamental pepper. Acta Horticulturae. 953: 298-304. https://doi.org/10.17660/actahortic.2012.953.41
- Nascimento NFF, Rêgo ER, Nascimento MF, Bruckner CH, et al. (2014). Combining ability for yield and fruit quality in the pepper Capsicum annuum. Genetics and Molecular Research. 13: 3237-3249. https://doi.org/10.1556/aagr.48.2000.4.7
- Nascimento MF, Nascimento NFF, Rêgo ER, Bruckner CH, et al. (2015). Genetic diversity in a structured family of six generations of ornamental chili peppers (Capsicum annuum). Acta Horticulturae. 1087: 395-401. https://doi.org/10.17660/actahortic.2015.1087.53
- Neitzke RS, Barbieri RL, Rodrigues WF, Carrêa IV, et al. (2010). Dissimilaridade genética entre acessos de pimenta com potencial ornamental. Horticultura Brasileira. 28: 47-53. https://doi.org/10.1590/s0102-05362010000100009
- Neitzke RS, Fischer SZ, Vasconcelos CS, Barbieri RL, et al. (2016). Pimentas ornamentais: aceitação e preferências do público consumidor. Horticultura Brasileira. 34: 102-109. https://doi.org/10.1590/s0102-053620160000100015
- Neto FVB, Leal NR, Gonçalves LSA, Filho LMR (2010). Descritores quantitativos na estimativa da divergência genética entre genótipos de mamoneira utilizando análises multivariadas. Revista Ciência Agronômica. 41: 294-299. https://doi.org/10.1590/s1806-66902010000200018
- Neto JJS, Rêgo ER, Nascimento MF, Filho VALS, et al. (2014). Variabilidade em população base de pimenteiras ornamentais (Capsicum annuum L.). Revista Ceres. 61:84-89. https://doi.org/10.1590/s0034-737x2014000100011
- Pastorinil LH, Romagnolo MB, Barbeiro C, Guerreiro RGO, et al. (2016). Germinação e crescimento inicial de Machaerium brasiliense Vogel (Fabaceae) em casa de vegetação. Floresta. 46: 2016. https://doi.org/10.5380/rf.v46i1.39625
- Pessoa AMS, Barroso PA, Rêgo ER, Medeiros GDA, et al. (2015a). Genetic divergence of physiological-quality traits of seeds in a population of peppers. Genetics and Molecular Research. 14: 12479-12488. https://doi.org/10.4238/2015.october.16.15
- Pessoa AMS, Rêgo ER, Barroso PA, Rêgo MM (2015b). Genetic Diversity and Importance of Morpho-Agronomic Traits in a Segregating F2 Population of Ornamental Pepper. Acta Horticulture. 1087: 195-200. https://doi.org/10.17660/actahortic.2015.1087.23
- Pickersgill B (1997). Genetic resources and breeding of Capsicum spp. Euphytica. 96: 129-133. https://doi.org/10.1007/978-3-319-06532-8_4
- Rao AV, Prasad ASR, Sai Krishna T, Sechu DV, et al. 1981). Genetic divergence among some brown plant hopper resistant rice varieties. The India Journal of Genetic Plant Breeding. 41: 179-185.
- Rao CR (1952). Advanced statistical methods in biometric research. John Wiley & Sons, New York. https://doi.org/10.1093/aibsbulletin/2.5.12-a
- Rêgo ER, Rêgo MM, Cruz CD, Finger FL, Amaral DSSL (2003). Genetic Diversity analysis of peppers: a comparison of discarding variables methods. Crop Breeding and Applied Biotechnology. 3: 19-26. https://doi.org/10.12702/1984-7033.v03n01a03
- Rêgo ER, Rêgo MM, Finger FL, Cruz CD, Casali VWD (2009). A diallel study of yield components and fruit quality in chilli peppers (Capsicum baccatum). Euphytica. 168: 275-287. https://doi.org/10.1007/s10681-009-9947-y
- Rêgo ER, Silva DFS, Rêgo MM, Santos RMC (2010). Diversidade entre linhagens e importância de caracteres relacionados à longevidade em vaso de linhagens de pimenteiras ornamentais. Revista Brasileira de Horticultura Ornamental. 16: 165-168. https://doi.org/10.14295/rbho.v16i2.558
- Rêgo ER, Rêgo MM, Matos IWF, Barbosa LA (2011a). Morphological and chemical characterization of fruits of Capsicum spp. accessions. Horticultura Brasileira. 29: 364-371. https://doi.org/10.1590/s0102-05362011000300018
- Rêgo ER, Rêgo MM, Cruz CD, Finger FL, Casali VWD (2011b). Phenotypic diversity, correlation and importance of variables for fruit quality and yield traits in Brazilian peppers (Capsicum baccatum). Genetic Resources and Crop Evolution. 58: 909-918. https://doi.org/10.1007/s10722-010-9628-7
- Rêgo ER, Nascimento MF, Nascimento NFF, Santos RMC, et al. (2012). Testing methods for producing self-pollinated fruits in ornamental peppers. Horticultura Brasileira. 30: 669-672.
- Rêgo ER, Rêgo MM, Finger FL (2015a). Methodological basis and advances for ornamental pepper breeding program in Brazil. Acta Horticulturae. 1087:309-314. https://doi.org/10.17660/actahortic.2015.1087.39
- Rêgo MM, Sapucay MJLC, Rêgo ER, Araújo ER (2015b). Analysis of Divergence and Correlation of Quantitative Traits in Ornamental Pepper (Capsicum spp.). Acta Horticulturae. 1087: 389-394. https://doi.org/10.17660/actahortic.2015.1087.52
- Rêgo ER, Rêgo MM (2016). Genetics and Breeding of Chili Pepper Capsicum spp. In: Rêgo ER, Rêgo MM, Finger FL (2016). Production and Breeding of Chilli Peppers (Capsicum spp.). Springer International Publishing Switzerland. 1-129.
- Rodriguês HCA, Carvalho SP, Carvalho AA, Filho JLSC (2010). Avaliação da diversidade genética entre acessos de mamoneira (Ricinus communis L.) por meio de caracteres morfoagronômicos. Revista Ceres. 57: 773-777.
- Rosado AM, Rosado TB, Júnior MFRR, Bhering LL (2009). Ganhos genéticos preditos por diferentes métodos de seleção em progênies de Eucalyptus urophylla. Pesquisa Agropecuária Brasileira. 44: 1653 – 1659.
- Rosado AM, Alves AA, Laviola BG, Bherin LL (2012). Seleção simultânea de clones de eucalipto de acordo com produtividade, estabilidade e adaptabilidade. Pesquisa Agropecuária Brasileira. 47: 964-971. https://doi.org/10.1590/s0100-204x2012000700013
- Santos ER, Barros HB, Ferraz EC, Cella AJS, et al. (2011). Divergência entre genótipos de soja, cultivados em várzea irrigada. Ceres. 58: 755-764. https://doi.org/10.1590/s0034-737x2011000600012
- Santos RMC, Nascimento NFF, Borém A, Finger FL, et al. (2013). Ornamental pepper breeding: could a chili be a flower ornamental plant? Acta Horticulturae. 1000: 451-456. https://doi.org/10.17660/actahortic.2013.1000.63
- Silva AR, Cecon PR, Rêgo ER, Nascimento M (2011). Avaliação do coeficiente de variação experimental para caracteres de frutos de pimenteiras. Revista Ceres. 58: 168-171.
- Silva CQ, Jasmim JM, Santos JO, Bento CS, et al. (2015). Phenotyping and selecting parents for ornamental purposes in pepper accessions. Horticultura Brasileira. 33: 066-073.
- Silva AR, Rêgo ER, Pessoa AMS, Rêgo MM (2016). Correlation network analysis between phenotypic and genotypic traits of chili pepper. Pesquisa Agropecuária Brasileira. 51: 372-377.
- Singh D (1981). The relative importance of characters affecting genetic divergence. Indian Journal of Genetics and Plant Breeding. 4: 237-245.
- Stähelin D, Valentini G, Andrade LRB, Verissimo MAA, et al. (2011). Screening multivariado entre acessos e cultivares de feijão do grupo preto para utilização em blocos de cruzamento. Biotemas. 24: 95-103.
- Stommel JR, Bosland P (2006). Ornamental pepper. Capsicum annuum. In: Anderson NO. Flower breeding and genetics: issues, challenges, and opportunities for the 21st Century, ed. Dordrecht, Holanda: Springer.561 – 599.
- Sudré CP, Rodrigues R, Riva EM, Karasawa M, et al. (2005). Divergência genética entre acessos de pimenta e pimentão utilizando técnicas multivariadas. Horticultura Brasileira. 23: 22-27.
- Ulhoa AB, Pereira TN, Silva RN, Ragassi CF, et al. (2014). Caracterização molecular de linhagens de pimenta do tipo Jalapeño amarelo. Horticultura Brasileira. 32: 35-40.
- Vasconcelos CS, Barbieri RL, Neitzke RS, Priori D, et al. (2012). Determinação da dissimilaridade genética entre acessos de Capsicum chinense com base em características de flores. Revista Ceres 59: 493-498.
- Vasconcelos ES, Cruz CD, Bhering LL, Júnior MFRR (2007). Método alternativo para análise de agrupamento. Pesquisa Agropecuaria Brasileira. 42: 1421-1428.
- Wang D, Bosland PW (2006). The Genes of Capsicum. Hortscience. 41:1169-1187.
Keywords:
Download:
Full PDF- Share This