Genetic structure and diversity of Senefeldera verticillata (Euphorbiaceae) in semideciduous seasonal forest fragments
Received: June 12, 2018
Accepted: August 07, 2018
Published: August 29, 2018
Genet.Mol.Res. 17(3): http://dx.doi.org/gmr18059
DOI: http://dx.doi.org/10.4238/gmr18059
Abstract
Senefeldera verticillata (Euphorbiaceae) is a species that exclusively occurs in the Atlantic Forest; it is used in the restoration and recovery of degraded areas and has therapeutic uses. Due to the scarcity of information on genetic patterns of this species, genetic diversity was characterized by the use of molecular markers, Inter Simple Sequence Repeats (ISSR). Leaf samples were collected from two populations located in the National Forest (FLONA) of Pacotuba and in the Private Reserve of Natural Patrimony (RPPN) Cafundó. Twelve ISSR primers were used, resulting in 179 amplification products, with 76% polymorphic bands. The genetic diversity values of Nei (H’) and Shannon index (I) were 0.329 and 0.503, respectively. The estimated historical gene flow among the fragments was high (Nm = 13.542). Molecular analysis of variance showed that most of the genetic diversity is within conservation units (95%), with genetic differentiation among populations considered moderate (ΦST = 0.0501). Thus we propose conservation of genetically dissimilar individuals in the two localities, so that the existing variability is preserved. Six groups were identified by the unweighted arithmetic means clustering method, in which 30 matrices of both fragments were collected in a single group. Bayesian analysis indicated that there is a small degree of genetic variation among populations, though organization of groups by locality was not confirmed by the STRUCTURE software. We were able to identify genetic divergence among the trees evaluated in these conservation units, demonstrating the usefulness of the ISSR markers. In addition, this information could help in the adoption of strategies for the selection of respresentative specimens to compose a seed bank of native forest species of the state of Espírito Santo
Introduction
The Atlantic Forest is known worldwide for its rich biodiversity and endemism. Among the states included in the Brazilian Atlantic Forest biome, the most representative is Espírito Santo, which has an area of 45,597 km2; this state initially had approximately 90% of its vegetation cover composed of this biome (Instituto Nacional de Pesquisas Espaciais, 2015). According to Assis et al. (2007), among the typologies that make up the Atlantic Forest in the state, are the semideciduous seasonal forests, which are the second most important vegetation formation in terms of occupied area, mainly concentrated in the south.
The conservation areas of Pacotuba National Forest (FLONA of Pacotuba) and the Private Reserve of Natural Patrimony Cafundó (RPPN Cafundó) are located in southern Espírito Santo state. They are significant remnants of the original forest in this region, being of fundamental importance for the conservation of these environments (Instituto de Pesquisas da Mata Atlântica, 2005).
These conservation units have a wide diversity of tree species, but the species Senefeldera verticillata (Euphorbiaceae), commonly known as Sucanga, stands out. It is anendemic species of Brazil and is only found in the Atlantic Forest in the states of Pernambuco, Bahia, Alagoas, Minas Gerais, Espírito Santo and Rio de Janeiro. It is a pioneer tree, monoecious with a zoocoric dispersion syndrome (Pscheidt and Cordeiro, 2012). It presents rapid growth and easy propagation, allowing its use in the restoration and recovery of degraded areas, besides being economically important for therapeutic purposes (Hans, 2012; Pires et al., 2013).
Despite it’secological and economic potential, this species is still little studied; so far there is no information about diversity and / or genetic structure.
The genetic variability of a species is the basis for its adaptation and survival, being the main factor that allows evolution to occur; even though it is amplified by evolutionary factors such as mutation and migration, it can be lost by genetic drift, mating between related individuals and selection (Cole, 2003). Knowledge about the genetic diversity of a species has been central to decision-making in the constitution of seed lots that will compose seedlings for genetic conservation and breeding (Sebbenn, 2006).
Currently, there is a wide variety of molecular markers available that allow the analysis of genetic variability among and within populations. Among these markers, we highlight ISSRs (Inter Simple Sequence Repeats) (Zietkiewicz et al., 1994), characterized as dominant and semiarbitrary (Souza et al., 2005). These markers are increasingly used in evaluations of genetic diversity of tree species, as they are inexpensive, highly reproducible and informative (Nybom, 2004; Kumar et al., 2011).
We evaluated the pattern of genetic diversity in S. verticillata trees located in two reserves in Espírito Santo state by means ISSR markers, to help define strategies suitable for their preservation. This data will help select trees as future seed providers to help recover native forests of this state.
Material and Methods
Population Sampling and Vegetable Material
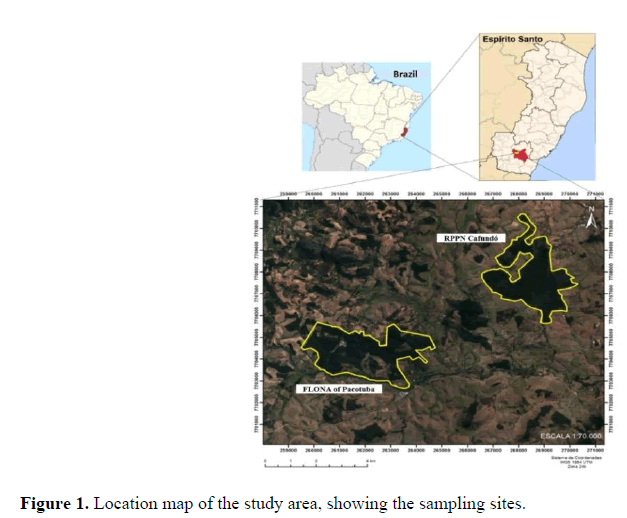
Young leaves with good phytosanitary appearance were randomly collected from 39 adult S. verticillata trees; 17 trees were located in the FLONA of Pacotuba (20º45 ' South latitude and 41º17' West longitude) and 22 were located in the RPPN Cafundó (20º43'latitude South and 41º13'west longitude) (Fig. 1). Sampling took into account the population distribution, with at least 50 m distance between the trees, in order to avoid the collection of related individuals.
Extraction and quantification of genomic DNA
Genomic DNA was extracted by the protocol of Doyle and Doyle (1990), with modifications proposed by the Instituto Agronômico de Campinas (IAC), adjusting the concentrations of 1% polyvinylpyrrolidone (PVP) and cetyl trimethylammonium bromide (CTAB) to 2%. The concentration and purity of the samples were analyzed by spectrophotometry using the NanoDrop 2000 apparatus (Thermo Fisher Scientific, Waltham, MA, USA). As a parameter of quality of the samples, the criterion described by Barbosa (1998), which based on the relation A260 / A280, was adopted; it states that ratios lower than 1.8 indicate protein contamination and higher than 2.0 indicate contamination with chloroform or phenol.
PCR amplification
Twelve ISSR primers produced by the University of British Columbia, Vancouver, Canada, were used for the genetic characterization of S. verticillata individuals. Polymerase chain reactions (PCR) were performed with a total reaction volume of 20 μl, containing 1X buffer (10 mM Tris-HCl pH 8.5 and 50 mMKCl), 2.5 mM MgCl2, 0.25 mM each dNTP, 0.2 μM primer, 1 unit Taq DNA polymerase and 50 ng genomic DNA.
The amplifications were conducted in a thermal cycler (Applied Biosystems, Veriti model) under the following conditions: initial denaturation of 5 min at 94°C, followed by 35 cycles of denaturation (94°C for 45 sec), annealing (52°C for 45 sec) and elongation (72°C for 90 sec) and a final extension of 72°C for 7 min.
The amplification products were separated by electrophoresis in 2% agarose gel in 1X TBE (Tris 0.089 mol-L -1, boric acid 0.089 mol-L -1 and EDTA 0.002 mol-L -1) for 5 h at 100 V (0.50 μg/ mL-1) and photographed under UV light (ChemiDoc MP Image System - Bio Rad), allowing visualization of the amplification pattern of each primer, and the molecular size of the fragments, estimated with a 100 bp molecular weight marker (Ladder).
Statistical analysis
The information contained in the gels was converted into binary matrices, being determined by presence (1) and absence (0) of bands in each locus. Afterwards, a descriptive analysis of the data was carried out, which included as parameters the total number of bands (TNB), number of polymorphic bands (NPB), percentage of polymorphic bands (PPB) per primer and size variation of fragments generated in pairs of bases (VFG).
After the descriptive analyzes, the POPGENE software version 3.2 (Yeh and Boyle, 1997) was used to calculate the following parameters of genetic diversity: number of effective alleles (Ne), percentage of polymorphic loci (PPL), Shannon diversity index (I) (Shannon and Weaver, 1949) and genetic diversity of Nei (H’) (Nei, 1973). The genetic differentiation coefficient (GST) and gene flow (Nm) were also calculated with the aid of this software.
Using the GENES program (Cruz, 2013), a similarity matrix was calculated between individuals, which was converted to a dissimilarity matrix using the arithmetic complement of the Jaccard coefficient (Jaccard, 1901), and a dendrogram was constructed by the method unweighted arithmetic means (UPGMA). The cut-off point for the dendrogram was determined by the method proposed by Mojema (1977). The cophenetic correlation coefficient (r) was calculated in order to check the consistency of the cluster.
An analysis of molecular variance (AMOVA) was used to evaluate the distribution of genetic variability among and within populations, as well as to infer their structure, using ARLEQUIN software version 3.11 (Excoffier et al., 1992).
The STRUCTURE 2.3 program (Pritchard et al., 2000), based on Bayesian statistics was used to infer the number of groups (k). Twenty runs were performed for each value of K, where the number of established groups (K) ranged from K = 1 to K = 5, with 250,000 burn-ins and 1,000,000 Markov Chain Monte Carlo (MCMC) simulations. For the definition of the most probable K value, we used the mean and standard deviation of each probability value of K and the ΔK method proposed by Evanno et al. (2005), using the software STRUCTURE HAVESTER (Earl and Von Holdt, 2012).
Results and Discussion
The twelve primers used allowed us to obtain 179 amplified fragments, of which 76% were polymorphic. The use of ISSR primers in this study was satisfactory for the detection of polymorphism in natural populations of S. verticillata, where the percentage value of polymorphic bands can be compared with results in similar works.
In a study of genetic diversity in wild populations of Eugenia uniflora in forest remnants with different successional stages, 79% polymorphic loci were found in the areas of advanced succession stage and 70% in the area of initial succession (Aguiar et al., 2013). In another study involving the use of ISSR markers with the tree species Schizolobium parahyba var. amazonicum, 136 loci were found, 58% being polymorphic (Silva Júnior et al., 2017), fewer than in our study.
The number of bands per primer varied between 8 and 24, with a mean of 14.9 bands per primer, with a variation of molecular weights of the different loci, from 150 to 2080 base pairs (bp). Primer UBC 818 revealed the largest number of polymorphic bands (20), whereas the primer with the least number of polymorphic bands was UBC 842, with three bands (Table 1).
| Primer | Sequence | TNB | NPB | PP;B % | VFG (min-max) |
|---|---|---|---|---|---|
| UBC 807 | (AG)8T | 13 | 11 | 84.61 | 150 – 1600 |
| UBC 808 | (AG)8C | 18 | 15 | 83.33 | 250 – 2080 |
| UBC 811 | (GA)8C | 13 | 10 | 76.92 | 250 – 1520 |
| UBC 812 | (GA)8ª | 17 | 10 | 58.82 | 240 – 2080 |
| UBC 818 | (CA)8G | 24 | 20 | 83.33 | 360 – 2080 |
| UBC 836 | (AG)8YA | 9 | 5 | 55.55 | 260 – 2080 |
| UBC 842 | (GA)8YG | 8 | 3 | 37.50 | 250 – 2080 |
| UBC 856 | (AC)8YA | 17 | 13 | 76.47 | 480 – 2080 |
| UBC 864 | (ATG)5 | 16 | 14 | 87.50 | 280 – 1500 |
| UBC 868 | (GAA)6 | 17 | 13 | 76.47 | 300 – 2080 |
| UBC 880 | (GGAGA)3 | 18 | 16 | 88.88 | 230 – 1020 |
| UBC 891 | HVH(TG)7 | 9 | 6 | 66.66 | 260 – 1220 |
| TOTAL | 179 | 136 | 75.97 | - |
*A = Adenine; T = Thymine; C = Cytosine; G = Guanine; H = (A, T or C); V = (A, C or G) and Y = (C or T).
Table 1. ISSR primers used in the study of Senefeldera verticillata, including total number of bands (TNB), number of polymorphic bands (NPB), percentage of polymorphic bands per primer (PPB) and size variation of fragments generated in pairs of bases (VFG).
At the species level, the genetic diversity measured by number of effective alleles (Ne), genetic diversity of Nei (H’) and Shannon (I) index were 1.538; 0.329 and 0.503, respectively. Based on the H’ and I calculations, the genetic variations of the two populations classified them as FLONA> RPPN; however, there were no large differences in the levels of diversity between the two fragments (Table 2).
| Population | N | Ne | H’ | I | PPL(%) | |
| FLONA of Pacotuba | 17 | 1.544 | 0.327 | 0.498 | 75.98 | |
| RPPN Cafundó | 22 | 1.501 | 0.310 | 0.478 | 75.98 | |
| Species | 39 | 1.538 | 0.329 | 0.503 | 75.98 |
N: number of samples; Ne: number of effective alleles; H’: genetic diversity of Nei; I: Shannon index. PPL: percentage of polymorphic loci.
Table 2. Genetic variation in populations of Senefeldera verticillata detected by ISSR markers.
The value of I can vary between 0 and 1, and values equal to 1 indicate the maximum diversity observed in a population (Pádua and Ferreira, 2008). Under natural conditions, the value of H’ is never zero, due to the frequent incorporation of new alleles by crosses, even in small populations or fragmented environments, as well as by losses caused by genetic drift (Silva et al., 2014).
Based on ISSR markers in this research, when compared to other works with tree species and using a similar methodology, diversity in S. verticillata can be considered high. Research on the species Myrciaria tenella (Myrtaceae) located in the Reserve of the Natural Patrimony in the area of restinga, in the municipality of Caju-SE, found I= 0.52 and H’= 0.35, considered as relevant diversity levels (Santana et al., 2016). In another study involving the diversity and genetic structure of a natural population of Nectandra megapotamica, located in the Atlantic Forest area, values very close to the results of our study were found for I, H’ and percentage of polymorphic loci using RAPD markers (Costa et al., 2015).
The results we found for genetic diversity may be related to the characteristics of the tree species and its location. Because it is a long life species and in a preservation area, where selective cutting is not allowed, habitat fragmentation apparently was not enough to affect the existing genetic diversity. Another factor that may contribute to the maintenance of the species is the presence of fauna, especially birds, which promote seed dispersal.
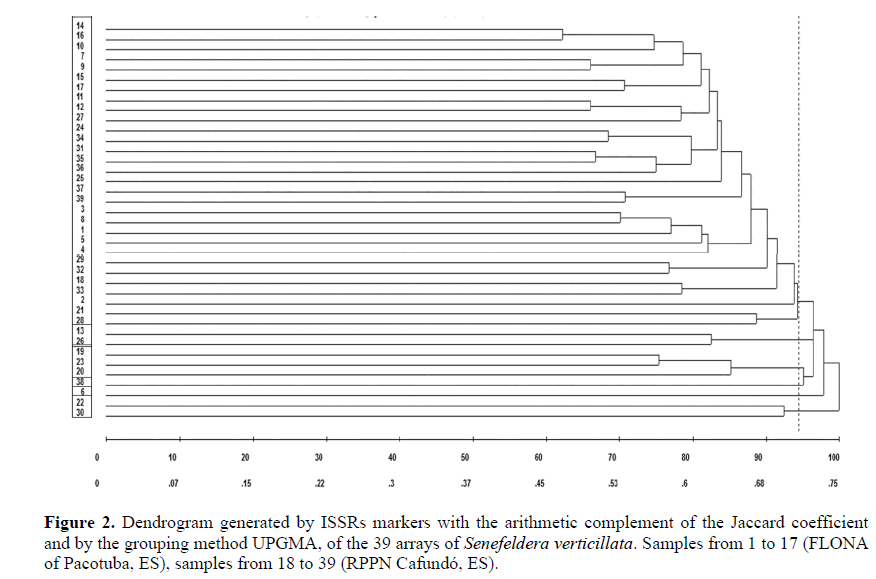
Dissimilarity between matrices based on ISSR markers ranged from 0.47 to 0.90. The highest dissimilarity value (0.90) was observed between matrices 6 and 22, while the lowest (0.47) occurred between matrices 14 and 16, with the four matrices belonging to the FLONA of Pacotuba.
Based on hierarchical grouping (UPGMA) of dissimilarity values and taking as a cut-off the percentage 94.46%, it was possible to identify six groups (Fig. 2).
The matrices 38 and 6 were positioned in distinct groups, four and five, respectively, indicating a greater genetic distinction between these individuals. The pairs, matrices 13-26 and 22-30, formed groups two and six, respectively. Matrices 19, 23 and 20 were pooled to form group three, and finally group one was formed by 30 matrices, which encompassed both individuals belonging to the FLONA of Pacotuba and the RPPN Canfundó. By evaluation of the dendrogram constructed by the grouping of the matrices, we perceived greater variability within the forest fragments than among them, since there was no predominance in the formation of groups containing individuals distributed by forest fragment.
The correlation coefficient was r = 0.71, revealing a high association between the similarities obtained by the Jaccard coefficient (dissimilarity matrix) and those represented in the dendrogram (cophenetic matrix). The higher the correlation value, the less distortion caused by the cluster (Cruz et al., 2011).
Other analyzes such as (GST) and gene flow (Nm) were also performed. The data (GST = 0.035; Nm = 13.542) demonstrates intense gene flow between the two populations, decreasing the differences between them, which is evident in the dendrogram by formation of group one, which gathered individuals from both forest fragments.
The analysis of molecular variance (AMOVA) indicated that 5.01% of the total variance is between populations and 94.99% within populations, demonstrating that the greatest genetic variation is in the intrapopulation component (Table 3). This result could reflect intense historical gene flow between the units.
| Source of variation | DF | Sum of squares | Components of variance | Change (%) | |
| Between fragments | 1 | 55.630 | 1.47551 | 5.01 | |
| Within the fragments | 37 | 1005.056 | 27.92171 | 94.99 | |
| Total | 38 | 1060.685 | 29.39723 | ||
| ΦST= 0.0501 |
DF = Degrees of freedom
Table 3. Molecular variance analysis of39 individuals of Senefeldera verticillata in two forest fragments in Espírito Santo state.
In allogamous species or mixed breeding systems, in which allogamy predominates, genetic variation is concentrated within the populations and less variation occurs between them; divergence between populations will be smaller the greater the gene flow (Loveless and Hamrick, 1987). Our results are similar to what has been generally found for natural populations of tropical tree species; most of the genetic diversity is present within populations (Gomes et al., 2011; Rossi et al., 2014). As S. verticillata is characterized as being a typically allogenic species, the results of variability that we found are in line with that expected.
The coefficient of relative diversity between groups (GST) was 0.0356, indicating that 3.56% of the total genetic variation occurs among populations. This result is comparable to the population variance provided by AMOVA. The low value of ΦST found (0.0501) indicates that there is no structuring between the populations; that is gene flow between them has been high. Although the two forest fragments are not isolated by a large geographic distance, it is worth mentioning that this species is a tree with a long life cycle, and since adult trees were selected for evaluations, the estimation of genetic divergence reflects historical gene flow.
The results of Nm and intrapopulation genetic variability obtained in this work indicate that, even though FLONA and RPPN do not form a continuous landscape, habitat fragmentation does not significantly affect the genetic diversity of S. verticillata in these environments. Wright (1978) considered that among the subpopulations differentiation is not considered insignificant, even if the FST values are equal to or less than 0.05. Thus, the result found for ΦST, should be considered in decisions taken to preserve conservation units.
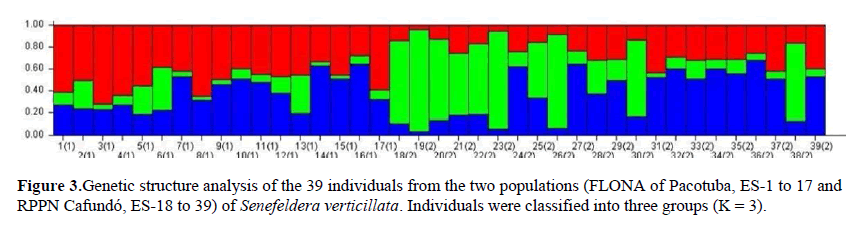
From the simulations performed by the STRUCTURE 2.3 program using the Bayesian approach, we observe the division of S. verticillata matrices belonging to the FLONA of Pacotuba (samples from 1 to 17) and RPPN Cafundó (samples from 18 to 39) into three genetic groups, making the presence of a mixture of genetic material between the matrices clear (Fig. 3).
Through the graphic visualization of the population structure, it is possible to observe that there was no large separation of genetic groups per conservation unit, which indicates that there is no great genetic difference among the fragments, as already evidenced in previous analyses. However, it is possible to observe a contrast in the distribution of the green group (Fig. 3), more prominent in the population of the RPPN Cafundó, and of the red group, which was more accentuated in the population of the FLONA of Pacotuba, indicating a certain differentiation between the two localities. This fact was confirmed by the result of ΦST (0.0501), which revealed moderate genetic differentiation between the units.
These results are also in agreement with the AMOVA data, which indicated that most of the genetic differentiation is at the intrapopulational level, as well as the gene flow value found (Nm = 13.542), which is responsible for the exchange of genes among the forest fragments, a process by which genetic variability is maintained among S. verticillata individuals in this area.
Conclusion
The percentage of polymorphism detected by the ISSR marker in S. verticillata indicated that the primers used in this study were adequate for the analyses of genetic diversity and will aid in the preservation work of this species. Based on AMOVA and GST, moderate genetic differentiation was observed among conservation units. Due to the greater genetic variability found within the forest fragments, the preservation of several individuals in both conservation units is recommended, in order to maintain the existing intrapopulation genetic variability.
Acknowledgments
We thank the Fundação de Amparo a Pesquisa do Espírito Santo (FAPES) - EDITAL FAPESNº 11/2013 - Research Applied to State Public Policies - Theme: Research in Agriculture in the State of Espírito Santo - Process no. 65766261/14, the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for granting the scholarship, the Universidade Federal do Espírito Santo (UFES) for the lab space and the Núcleo de Pesquisa Científica e Tecnológica em Meio Ambiente, Silvicultura e Ecologia (NUPEMASE) for their support.
About the Authors
Corresponding Author
A.L. Silva Júnior
Laboratório de Bioquímica e Biologia Molecular, Programa de Pós-Graduação em Genética e Melhoramento, Centro de Ciências Agrárias e Engenharias, Universidade Federal do EspÃÂrito Santo, Alegre, ES, Brasil
- Email:
- adelsonlemes@yahoo.com.br
References
- Aguiar RV, Cansian RL, Kubiak GB, Slaviero LB, et al. (2013). Variabilidade genética de Eugenia uniflora L. em remanescentes florestais em diferentes estádios sucessionais. Rev. Ceres. 60: 226-233. Available at [http://dx.doi.org/10.1590/S0034-737X2013000200011].
- Assis AM, Magnago LFS, Fernandes HQB (2007). Florestaestacional semidecidual de terras baixas, submontana e montana. In: Espécies da Flora Ameaçada de Extinção no Estado do Espírito Santo (Simonelli M, Fraga CN, eds.).Instituto de Pesquisas da Mata Atlântica, Vitória.
- Barbosa MM (1998). Quantificação e controle da qualidade do DNA genômico. In: Marcadores moleculares em plantas (MILACH, S). Porto Alegre.
- Cole TC (2003). Genetic variation in rare and common plants. Annu. Rev. Ecol. Evol. Syst.34: 213-237. Available at [https://doi.org/10.1146/annurev.ecolsys.34.030102.151717].
- Costa LS, Reinige LRS, Heinzmann BM, Amaral LP, et al. (2015). Study of the genetic diversity and structure of a natural population of Nectandramegapotamica (Spreng.) Mez. using RAPD markers. Genet. Mol. Res. 14: 18407-18413. Available at [http://dx.doi.org/10.4238/2015.December.23.28].
- Cruz DT,Schnadelbach AS, Lambert SM, Ribeiro PL, et al. (2011). Genetic and morphological variability in Cattleyaelongata Barb. Rodr. (Orchidaceae), endemic to the campo rupestre vegetation in NotheasternBrazil. Plant. Syst.Evol. 294: 87-98.
- Cruz CD (2013). GENES - a software package for analysis in experimental statistics and quantitative genetics. Acta Sci. Agron. 35: 271-276. Available at [http://doi.org/10.4025/actasciagron.v35i3.21251].
- Doyle JJ and Doyle JL (1990). Isolation of plant DNA from fresh tissue. Focus. 12: 13-15.
- Earl DA and Vonholdt BM (2012). Structure Harvester: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genet. Resour. 4: 359-361. Available at [http://doi.org/10.1007/s12686-011-9548-7].
- Excoffier L, Smouse PE and Quattro JM (1992). Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics. 131: 479-491. Available at [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1205020/].
- Evanno G, Regnaut S andGoudet, J (2005). Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol. Ecol. 14: 2611-2620.Available at [http://doi.org/10.1111/j.1365-294X.2005.02553.x].
- Gomes LRP, Lopes MTG, Bentes JLDS, Barros WS, et al. (2011). Genetic diversity in natural populations of Buriti (Mauritia flexuosa L. f.). Crop Breed.Appl.Biotechnol.11: 216-223. Available at [http://dx.doi.org/10.1590/S1984-70332011000300003].
- Hans JE (2012). The tribe Hippomaneae (Euphorbiaceae) in Brazil: A tribo Hippomaneae (Euphorbiaceae) no Brasil. Rodriguésia63: 209-225. Available at [http://dx.doi.org/10.1590/S2175-78602012000100013].
- Instituto de Pesquisas da Mata Atlântica (2005). Conservação da Mata Atlântica no Estado do Espírito Santo: Cobertura florestal e unidades de conservação (Programa Centros para a Conservação da Biodiversidade – Conservação Internacional do Brasil). Vitória
- Instituto Nacional de Pesquisas Espaciais - INPE (2015). Atlas dos remanescentes florestais da Mata Atlântica período 2013-2014. São Paulo. Available at [http://www.inpe.br/noticias/noticia.php?Cod_Noticia=3891].
- Jaccard P (1901). Etude comparative de la distribuition floraledansuneporion des Alpes et des Jura. Bull.Soc.Vaud.eSci.Nat.37: 547-579. Available at [https://doi.org/ 10.5169/seals-266450].
- Kumar R, Dwivedi N, Singh RK and Kumar S (2011). A review on molecular characterization of pepper for Capsaicin and Oleoresin. Intl. J. Plant Breed. 5: 99-110. Available at [https://doi.org/ 10.3923/ijpbg.2011.99.110].
- Loveless MD and Hamrick JL (1987). Distribution de la variation geneticsenespéciesarbreastropicales. Ver. Biol. Trop.35:165-176.
- Mojema R (1977). Hierarchical grouping methods and stopping rules: an evaluation. Comput. J. 20: 359-363. Available at [https://doi.org/10.1093/comjnl/20.4.359].
- Nei M (1973). Analysis of gene diversity in subdivided populations. Proc. Natl. Acad. Sci.70: 3321-3323. Available at [https://www.ncbi.nlm.nih.gov/pubmed/4519626].
- Nybom H (2004). Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol. Ecol. 13: 1143-1155. Available at [https://www.ncbi.nlm.nih.gov/pubmed/15078452].
- Pádua JG and Ferreira FR (2008). Recursos genéticos de fruteiras. In: Fundamentos do melhoramento de fruteiras (Bruckner CH, ed.).Editora UFV, Viçosa.
- Pires JPA, Silva AG and Freitas L (2013). Plant size, flowering synchrony and edgeeffects: What, how and where they affect the reproductive success of a Neotropical tree species. Austral Ecol. 39: 328-336. Available at [https://doi.org/10.1111/aec.12082]
- Pritchard JK, Stephens M and Donnelly P (2000). Inference of Population Structure Using Multilocus Genotype Data. Genetics. 155: 945-959. Available at [https://www.ncbi.nlm.nih.gov/pubmed/10835412].
- Pscheidt AC andCordeiro I (2012). Sinopse da tribo Hippomaneae (Euphorbiaceae) no Estado de São Paulo, Brasil. Hoehnea. 39: 347-368. Available at [http://dx.doi.org/10.1590/S2236-89062012000300001].
- Rossi FS, Rossi AAB, Dardengo JFE, Brauwers LR, et al. (2014). Diversidade genética em populações naturais de Mauritia flexuosa L. f. (Arecaceae) com uso de marcadores ISSR. Sci. For. 42: 631-639. Available at [http://www.ipef.br/publicacoes/scientia/nr104/cap17.pdf]
- Santana JGS, Nascimento ALS, Costa TS, Almeida TMB, et al.(2016). Estimation of genetic diversity in a natural population of cambui tree (MyrciariatenellaO. Berg) using ISSR markers. Genet. Mol. Res. 15(4). doi: 10.4238/gmr.15048819.
- Sebbenn AM (2006). Sistema de reprodução em espécies arbóreas tropicais e suas implicações para a seleção de árvores matrizes para reflorestamentos ambientais. Pomares de sementes de espécies florestais nativas. FUPEF, Curitiba.
- Shannon CE and Weaver WA (1949). Mathematical model of communication. Urbana: University of Illinois Press.
- Silva, AVC, Freire KCS, LédoAdaS and Rabbani ARC (2014). Diversity and genetic structure of jenipapo (Genipaamericana L.). Brazilian acessions. Sci. Agric. 71: 345-355. Available at [http://dx.doi.org/10.1590/0103-9016-2014-0038].
- Silva Júnior AL, Souza LC, Pereira AG, Caldeira MVW, et al. (2017). Genetic diversity of Schizolobium parahyba var. amazonicum (Huber ex. Ducke) Barneby, in a forest area in Brazil. Genet. Mol. Res. 16(3).doi:10.4238/gmr16039415
- Souza VQ, Pereira AS, Marinikopp M, Coimbra JLM, et al. (2005). Dissimilaridade genética em mutantes da aveia tolerantes e sensíveis a ácidos orgânicos. Bragantia. 64: 569-575. Available at [http://dx.doi.org/10.1590/S0006-87052005000400006].
- Wright S (1978). Evolution and genetics of populations. University of Chicago Press, Chicago.
- Yeh FC and Boyle TJB (1997). Population genetic analysis of co-dominant and dominant markers and quantitative traits. Belg J Bot. 29:157-163.
- Zietkiewicz E, Rafalski A andLabuda D (1994). Genome Fingerprinting by simple sequence repeat (SSR) - anchored polymerase chain reaction amplication. Genomics. 20: 176-183. Available at [https://www.ncbi.nlm.nih.gov/pubmed/8020964
Keywords:
Download:
Full PDF- Share This