Heat stress: impacts on fertility, productivity, and protein content of wheat cultivars
Received: October 14, 2017
Accepted: November 08, 2017
Published: December 15, 2017
Genet.Mol.Res. 16(4): gmr16039847
DOI: 10.4238/gmr16039847
Abstract
The aim of this study was to analyze the effects of high temperatures on ear fertility, yield components and protein content of wheat cultivars. Therefore, 96 genotypes were subjected to two environments in a greenhouse: control and heat stress. Each environment had two replicates, totalling 192 plots. The evaluations were: number of ear per plant, number of ear per pot, ear weight, number of grains per ear, number of sterile spikelet’s, number of viable grains, grain weight and protein content. Principal component analysis (PCA) and genetic and phenotypic correlations were performed. The results showed a significant drop in productivity of the genotypes subjected to heat stress as well as significant increase in the number of sterile spikelet’s. The PCA allowed the selection of nine promising genotypes for cultivation in environments with high temperatures with basis on these traits, especially the cultivars CD 104, CD 122 and TBIO Itaipu
Introduction
Wheat (Triticum aestivum) is an annual monocot, from the Middle East, belonging to the botanical family Poaceae, which includes all grasses. It is considered a basic cereal to civilization, as it is one of the most cultivated crops in the world. Although it presents significant numbers, domestic production in Brazil does not meet domestic demand. In addition to consumption, Brazil has imported about seven million of tons of the grain annually (Conab, 2017). To reach self-sufficiency in production, it is important to find alternatives for producing areas to ensure production stability. In this context, part of Brazil's Cerrado is a great option for the expansion of wheat crop production, either under the rainfed or irrigated condition (Conde et al., 2013).
According to Souza and Ramalho (2001), the existence of heat-tolerant cultivars is essential for the success of the wheat crop in this region. Since the species has a genetic base with available variability, cultivars adapted to high temperatures should be obtained for the expansion of cultivation in non-traditional areas (Souza and Pimentel, 2013), which is one of the main goals of wheat breeding programs in Brazil (Machado et al., 2010). Knowledge of the genetic parameters of the evaluated traits and understanding their interrelations are fundamental to the design of breeding programs and the selection process. Thus, the principal component analysis and the analysis of genetic and phenotypic correlations should help in the interpretation of the relationship among the variables and consequently aid in decision-making. This would allow the identification and selection of the most promising genotypes for cultivation and breeding, and to evaluate the relative importance of traits in the total variation among available genotypes (Moreira et al., 2009). Therefore, the objective of this study was to quantify and analyze the effects that high temperatures cause to ear fertility, grain yield and protein content of wheat genotypes and to identify genotypes that underwent less heat impact, to be used on breeding programs for cultivation in non-traditional areas.
Materials and Methods
Were evaluated 96 wheat genotypes (Table 1), most of which have been developed for the wheat growing Regions 3 and 4 (hot and dry) in the states of Paraná (PR), Mato Grosso do Sul (MS), Mato Grosso (MT), Minas Gerais (MG) and Goiás (GO). These genotypes were cultivated in two environments under controlled conditions (greenhouse): the first, called "Heat" (stress condition), was carried out by temperature control (night/daytime) between 25°C/35°C. The second, called "Control", was conducted in temperatures at C 15ºC/25ºC throughout the cycle (night/daytime).
| No. | Genotype | No. | Genotypes | No. | Genotypes | |
|---|---|---|---|---|---|---|
| 1 | Anahuac 75 | 33 | CD | 118 | 65 | IPR 85 |
| 2 | BH 1146 | 34 | CD | 122 | 66 | IPR Catuara TM |
| 3 | BR 18 Terena | 35 | CD | 123 | 67 | MGS Ágata |
| 4 | BR 23 | 36 | CD | 124 | 68 | MGS Aliança |
| 5 | BR 24 | 37 | CD 1252 | 69 | MGS Brilhante | |
| 6 | BR 42 - Nambiquara | 38 | CD 1440 | 70 | Mirante | |
| 7 | BRS 120 | 39 | CD | 150 | 71 | Nesser |
| 8 | BRS 207 | 40 | CD | 151 | 72 | Ônix |
| 9 | BRS 208 | 41 | CD | 154 | 73 | PF 020037 |
| 10 | BRS 210 | 42 | CD 1550 | 74 | PF 050667 | |
| 11 | BRS 220 | 43 | BRS 394 | 75 | PF 080491 | |
| 12 | BRS 229 | 44 | CPAC 07340 | 76 | PF 080492 | |
| 13 | BRS 248 | 45 | CPAC 07434 | 77 | PF 090547 | |
| 14 | BRS 254 | 46 | CPAC 0770 | 78 | PF 100332 | |
| 15 | BRS 264 | 47 | Embrapa 21 | 79 | PF 100334 | |
| 16 | BRS 296 | 48 | Embrapa 22 | 80 | PF 100368 | |
| 17 | BRS 327 | 49 | Embrapa 42 | 81 | PF 100409 | |
| 18 | BRS 49 | 50 | Fundacep Bravo | 82 | BRS 404 | |
| 19 | BRS Gaivota | 51 | Fundacep Cristalino | 83 | PF 100838 | |
| 20 | BRS Gralha Azul | 52 | Fundacep Horizonte | 84 | PF 100857 | |
| 21 | BRS Guabijú | 53 | IAC 24 Tucuruí | 85 | PF 100860 | |
| 22 | BRS Guamirim | 54 | IAC 350 | 86 | PF 100936 | |
| 23 | BRS Pardela | 55 | IAC 370 Armagedon | 87 | Quartzo | |
| 24 | BRS Parrudo | 56 | IAC 375 | Parintins | 88 | Supera |
| 25 | BRS Tangará | 57 | IAC 380 Saira | 89 | TBIO Bandeirante | |
| 26 | CD 104 | 58 | IAC 381 Kuara | 90 | TBIO Iguaçú | |
| 27 | CD 105 | 59 | IAC 5 Maringá | 91 | TBIO Itaipú | |
| 28 | CD 108 | 60 | IAPAR 78 | 92 | TBIO Mestre | |
| 29 | CD 111 | 61 | IPR 128 | 93 | TBIO Pioneiro | |
| 30 | CD 114 | 62 | IPR 130 | 94 | TBIO Sinuelo | |
| 31 | CD 116 | 63 | IPR 136 | 95 | Topázio | |
| 32 | CD 117 | 64 | IPR 144 | 96 | UFVT1 Pioneiro | |
Table 1. List of 96 wheat genotypes used in the greenhouse experiments.
The experimental design was a randomized block design (RBD) with two replications in each condition (heat and control). The two replicates of each treatment amounted to 192 pots per environment, where each pot made up an experimental plot. The data was obtained from the assessment of four individual plants in each pot, for the following traits:
1. Number of ears per pot (NEPot): After harvesting, the ears present in each pot were counted;
2. Number of ears per plant (NEPlnt): From the number of ears per pot, were estimated the average number of ears per plant, by dividing the value obtained by the number of plants per pot;
3. Number of viable grains (VG): The total number of viable grains (not hollow or wrinkled) per plot was obtained;
4. Grain weight (GW): Weight of all grain harvested in the plot in grams (g);
5. Protein content in grain (PC): The analysis of grain protein content was performed by determination of total nitrogen adapted from the Kjeldahl method (Galvani and Gaertner 2006). The nitrogen content was multiplied by 5.83 to express the protein as suggested by Orr and Watt (1957).
6. The following traits were analyzed by calculating the average of three ears.
7. Average weight of ears (EW): Whole ears harvested in each pot were weighed on an analytical scale to four decimal places (0.0001);
8. Number of spikelet’s per ear (SE): The ears taken from each plot were evaluated for the total number of spikelet’s;
9. Number of sterile spikelet’s (SS): The empty spikelet’s, i.e., without grain presence, were considered as sterile;
10. Number of grains per ear (NGE): Average grain produced by each sampled ear.
11. Principal Component Analysis (PCA) was performed using the software Statistica (Stat soft, 2010). Genetic and phenotypic correlations were obtained through Selegen software (Resende, 2006).
Results
In principal component analysis (PCA) for the heat environment, the first three eigenvalues generated were greater than 1, explaining 79.82% of the variance contained in the nine original variables. According to the criteria proposed by Kaiser (1958), only those eigenvalues above one is considered, because they generate components with a relevant amount of information from the original variables.
The first principal component (PC1) retained 39.87% of the original variance. The main variables that explained that retention of variance was: number of grains per ear (NGE), number of viable grains (VG), number of ears per plant (NEPlnt), number of ears per pot (NEPot), grain weight (GW) and protein content (PC), having correlation values with the main components of -0.67, -0.70, -0.69, -0.72, -0.93 and -0.61, respectively (Table 2). Were considered important variables that presented correlation values above 0.6, independent of the sign (Ferraudo, 2010).
| Variables | PC1 | PC2 | PC3 |
|---|---|---|---|
| EW | -0.54 | 0.34 | 0.65 |
| NGE | -0.67 | 0.43 | 0.49 |
| SS | 0.05 | -0.93 | 0.33 |
| VG | -0.70 | 0.04 | -0.12 |
| NEPlnt | -0.69 | -0.34 | -0.49 |
| SE | -0.33 | -0.71 | 0.61 |
| NEPot | -0.72 | -0.36 | -0.47 |
| GW | -0.93 | 0.06 | -0.02 |
| PC | 0.61 | -0.20 | 0.14 |
EW: Average Weight of Ears; NGE: Number of Grains per Ear; SS: Number Of Sterile Spikelet’s; VG: Number of Viable Grains; NEPlnt: Number of Ears per Plant; SE: Number of Spikelet’s; NEPot: Number of Ears per Pot; GW: Grain Weight; PC: Protein Content.
Table 2. Correlation between the variables and the principal components (PC) of PCA analysis for the Heat environment.
The second principal component (PC2) retained 21.83% of the variance and was explained by the variables number of sterile spikelet’s (SS) and number of spikelet’s (SE), with a correlation of -0.93 and -0.71, respectively (Table 2). The variables average ear weight (EW) (0.65) and number of spikelet’s per ear (SE) (0.61) explained the retention of 18.11% of the variance of PC3 (Table 2).
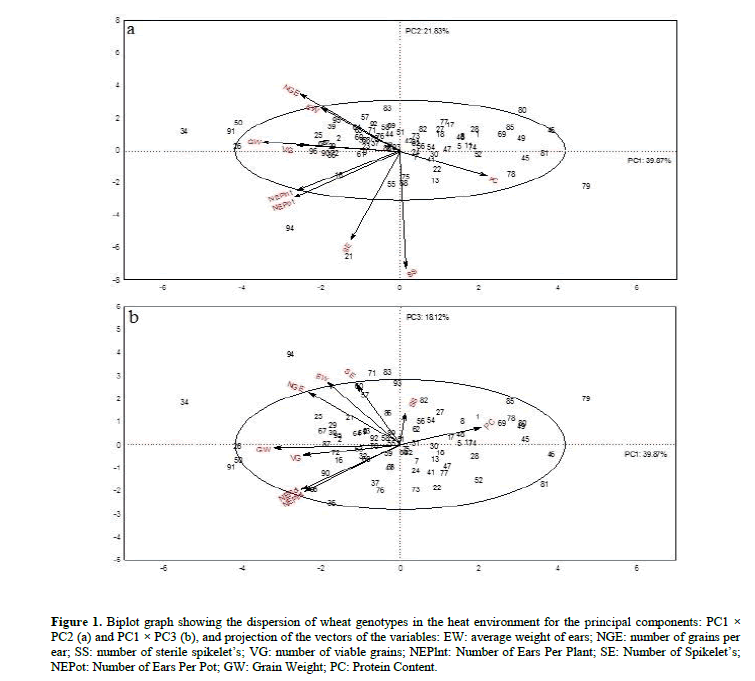
The two-dimensional plane formed by the PC1 (39.87%) and PC2 (21.83%) components retained a total of 61.70% of the original variance (Figure 1a) and was characterized by the variables: number of grains per ear (NGE) number of viable grains (VG), number of ears per plant (NEPlnt), number of ears per pot (NEPot), number of sterile spikelet’s (SS), number of spikelet’s (SE), grain weight (GW) and protein content (PC). It was possible to discriminate genotypes: BR Guabiju (21), CD 104 (26), CD 122 (34), Fundacep Bravo (50), PF 100 334 (79), TBIO Itaipú (91) and TBIO Sinuelo (94) by such traits whose averages are shown in Table 2.
Figure 1: Biplot graph showing the dispersion of wheat genotypes in the heat environment for the principal components: PC1 × PC2 (a) and PC1 × PC3 (b), and projection of the vectors of the variables: EW: average weight of ears; NGE: number of grains per ear; SS: number of sterile spikelet’s; VG: number of viable grains; NEPlnt: Number of Ears Per Plant; SE: Number of Spikelet’s; NEPot: Number of Ears Per Pot; GW: Grain Weight; PC: Protein Content.
The two-dimensional plane formed by PC1 (39.87%) and PC3 (18.11%) components retained 57.98% of the remaining variance (Figure 1b) and discriminated the same genotypes as the PC1 and PC2 two-dimensional plane, differing only in the discrimination of genotypes 71 - Nesser and 83 - PF 100838. The variables that stood out in PC3 were average ear weight (EW) and number of spikelet’s per ear (SE). The averages of the genotypes selected by principal component analysis are shown in Table 3.
| Genotype | EW (g) | SS (No.) | NGE (No.) | VG (No.) | NEPlnt (No.) | SE (No.) | NEPot (No.) | GW (g) | PC (%) |
|---|---|---|---|---|---|---|---|---|---|
| 21 | 0.70 | 68.36 | 3.67 | 3.04 | 3.91 | 80.43 | 14.50 | 4.47 | 12.77 |
| 26 | 1.16 | 11.53 | 5.30 | 63.49 | 3.75 | 40.05 | 10.00 | 9.47 | 11.85 |
| 34 | 1.50 | 12.45 | 5.95 | 255.52 | 3.49 | 47.81 | 10.50 | 7.94 | 10.91 |
| 50 | 0.98 | 3.16 | 5.35 | 220.77 | 3.16 | 31.60 | 11.00 | 8.09 | 9.97 |
| 71 | 1.16 | 14.09 | 5.81 | 16.05 | 1.57 | 47.81 | 6.50 | 5.60 | 13.24 |
| 79 | 0.56 | 40.76 | 2.89 | 0.00 | 0.87 | 48.86 | 3.00 | 0.60 | 16.54 |
| 83 | 1.41 | 5.84 | 5.55 | 8.05 | 1.32 | 36.59 | 5.50 | 5.18 | 13.11 |
| 91 | 0.97 | 5.96 | 5.14 | 272.54 | 3.12 | 32.10 | 12.50 | 8.24 | 10.89 |
| 94 | 0.88 | 63.94 | 5.24 | 192.4 | 2.32 | 92.29 | 9.50 | 5.79 | 12.05 |
EW: Average Weight of Ears; NGE: Number of Grains per Ear; SS: Number of Sterile Spikelet’s; VG: Number of Viable Grains; NEPlnt: Number of Ears per Plant; SE: Number of Spikelet’s; NEPot: Number of Ears per Pot; GW: Grain Weight; PC: Protein Content.
Table 3. Averages of the wheat genotypes selected by principal component analysis in the heat environment.
In the PCA control environment, three eigenvalues are greater than 1, explaining 76.88% of the variance contained in the original nine variables. The first principal component (PC1) retained 37.71% of the original variance and the main variables that explain this retention were: EW, NGE, VG, NEPlnt, SE, NEPot and GW, presenting correlation values with PC1 of -0.61, -0.63, -0.68, -0.71, -0.64, -0.75 and -0.75, respectively (Table 4). The second principal component (PC2), retained 25.16% of the original variance, where the main variables that were retained in this component were EW and SE, presenting correlations values -0.66 and -0.70 with PC2, respectively (Table 4). Principal component 3 retained 14.01% of the original variance and was characterized only by the variable SS, with 0.83 correlation (Table 4).
| Variables | PC1 | PC2 | PC3 |
|---|---|---|---|
| EW | -0.61 | -0.66 | -0.21 |
| NGE | -0.63 | -0.47 | -0.52 |
| SS | -0.14 | -0.44 | 0.83 |
| VG | -0.68 | 0.48 | -0.13 |
| NEPlnt | -0.71 | 0.59 | 0.16 |
| SE | -0.64 | -0.70 | 0.16 |
| NEPot | -0.75 | 0.56 | 0.17 |
| GW | -0.75 | 0.00 | 0.24 |
| PC | 0.31 | -0.12 | 0.32 |
EW: Average Weight of Ears; NGE: Number of Grains per Ear; SS: Number of Sterile Spikelet’s; VG: Number of Viable Grains; NEPlnt: Number of Ears per Plant; SE: Number of Spikelet’s; NEPot: Number of Ears per Pot; GW: Grain Weight; PC: Protein Content.
Table 4. Correlation between the variables and the principal components (PC) of PCA analysis for the Control environment.
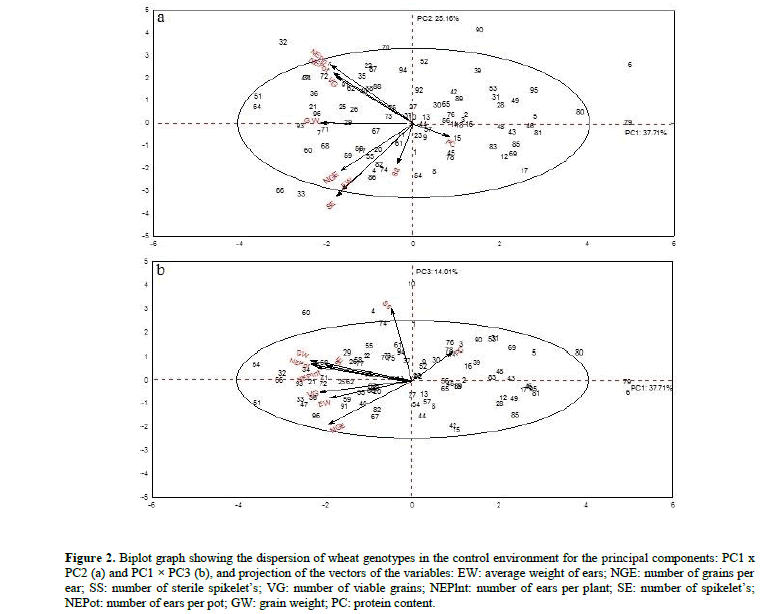
The two-dimensional plane formed by PC1 (37.71%) and PC2 (25.16%) components retained a total of 62.87% of the original variance and was characterized by the variables, EW, NGE, VG, NEPlnt, SE, NEPot, EW and GW, discriminating the genotypes CD 117 (32), CD 118 (33), Fundacep Cristalino (51), IPR 144 (64), IPR Catuara TM (66) and Mirante (70) (Figure 2a). The two-dimensional plane formed by PC1 and PC3 components retained 51.7% of the remaining variance and allowed discrimination of genotypes 51 - Fundacep Cristalino and 64 - IPR 144. (Figure 2b). The averages of the genotypes selected by principal component analysis are shown in Table 5.
Figure 2: Biplot graph showing the dispersion of wheat genotypes in the control environment for the principal components: PC1 x PC2 (a) and PC1 × PC3 (b), and projection of the vectors of the variables: EW: average weight of ears; NGE: number of grains per ear; SS: number of sterile spikelet’s; VG: number of viable grains; NEPlnt: number of ears per plant; SE: number of spikelet’s; NEPot: number of ears per pot; GW: grain weight; PC: protein content.
| Genotypes | EW (g) | SS (No.) | NGE (No.) | VG (No.) | NEPlnt (No.) | SE (No.) | NEPot (No.) | GW (g) | PC (%) |
|---|---|---|---|---|---|---|---|---|---|
| 32 | 1.30 | 6.29 | 28.41 | 406.82 | 6.50 | 35.22 | 18.00 | 16.28 | 11.73 |
| 33 | 2.31 | 13.39 | 41.15 | 216.44 | 2.75 | 57.16 | 11.00 | 12.74 | 10.90 |
| 51 | 1.66 | 5.42 | 37.33 | 346.77 | 4.50 | 43.47 | 18.00 | 19.10 | 10.83 |
| 64 | 1.78 | 14.57 | 30.41 | 386.90 | 4.25 | 45.43 | 17.00 | 19.94 | 10.39 |
| 66 | 2.28 | 18.05 | 39.00 | 188.62 | 3.13 | 57.97 | 12.50 | 14.44 | 10.08 |
| 70 | 1.04 | 9.27 | 19.18 | 322.11 | 4.25 | 29.21 | 17.00 | 15.72 | 11.19 |
EW: Average Weight of Ears; NGE: Number of Grains per Ear; SS: Number of Sterile Spikelet’s; VG: Number of Viable Grains; NEPlnt: Number of Ears per Plant; SE: Number of Spikelet’s; NEPot: Number of Ears per Pot; GW: Grain Weight; PC: Protein Content.
Table 5. Averages of the wheat genotypes selected by principal component analysis in the control environment.
The dispersion of 96 wheat genotypes (Figures 1 and 2) allows identification of genotypes with specific properties. The variables that have identified these genotypes were NEPlnt, EW, SE, NGE, NEPot and VG, and the direct selection for these traits is an effective strategy to increase wheat grain production. In the correlation analyses, were established that significant correlations are those above 0.6 independently of the sign. Were considered medium correlations those between 0.6 and 0.75 and high correlations above 0.75.
Both for the heat (Table 6) and for control (Table 7) environments, genetic and phenotypic correlations between NEPlnt and NEPot traits were high and in the same direction. The phenotypic and genotypic correlation between SE and SS for the heat environment was high and the same direction (Table 6), however, it was nonexistent in the control environment (Table 7). Genetic correlations of the trait viable grain (VG) with NEPot and NEPlnt were of high and the same direction, but the phenotypic correlations were of medium magnitude and the same direction, indicating that the environment acts in a negative way on the traits (Tables 6 and 7).
| Variables | NEPot | NEPlnt | EW | SE | SS | NGE | VG | GW | PC |
|---|---|---|---|---|---|---|---|---|---|
| NEPot | 1 | 0.82 | -0.06 | 0.16 | 0.08 | 0.09 | 0.46 | 0.69 | 0.12 |
| NEPlnt | 0.80 | 1 | -0.06 | 0.15 | 0.09 | 0.07 | 0.41 | 0.58 | -0.05 |
| EW | -0.05 | -0.06 | 1 | 0.21 | -0.17 | 0.75 | 0.10 | 0.48 | 0.01 |
| SE | 0.10 | 0.12 | 0.31 | 1 | 0.84 | 0.11 | 0.05 | 0.14 | 0.04 |
| SS | 0.04 | 0.08 | -0.17 | 0.80 | 1 | -0.41 | -0.15 | -0.18 | 0.02 |
| NGE | 0.09 | 0.04 | 0.78 | 0.23 | -0.39 | 1 | 0.38 | 0.59 | 0.02 |
| VG | 0.34 | 0.26 | 0.05 | 0.04 | -0.10 | 0.23 | 1 | 0.61 | 0.02 |
| GW | 0.70 | 0.55 | 0.45 | 0.15 | -0.18 | 0.54 | 0.49 | 1 | 0.09 |
| PC | -0.19 | -0.21 | -0.22 | 0.02 | 0.20 | -0.31 | -0.33 | -0.35 | 1 |
NEPot: Number of Ears Per Pot. NEPlnt: Number of Ears per Plant. EW: Average Weight of Ears. SE: Number of Spikelet’s. SS: Number of Sterile Spikelet’s. NGE: Number of Grains per Ear. VG: Number of Viable Grains. GW: Grain Weight. PC: Grain Protein Content.
Table 6. Genetic correlation matrix (above the diagonal) and phenotypic correlation matrix (below the diagonal) of the traits indicated as principal components in the heat environment.
| Variables | NEPot | NEPlnt | EW | SE | SS | NGE | VG | GW | PC |
|---|---|---|---|---|---|---|---|---|---|
| NEPot | 1 | 0.93 | 0.05 | 0.11 | -0.02 | 0.15 | 0.70 | 0.45 | -0.16 |
| NEPlnt | 0.91 | 1 | 0.02 | 0.05 | -0.08 | 0.12 | 0.69 | 0.41 | -0.16 |
| EW | 0.09 | 0.02 | 1 | 0.74 | 0.15 | 0.76 | 0.08 | 0.38 | -0.12 |
| SE | 0.11 | 0.04 | 0.71 | 1 | 0.54 | 0.70 | 0.08 | 0.36 | 0.02 |
| SS | -0.02 | -0.03 | 0.03 | 0.49 | 1 | -0.14 | -0.11 | 0.25 | 0.10 |
| NGE | 0.11 | 0.03 | 0.78 | 0.69 | -0.24 | 1 | 0.25 | 0.23 | -0.10 |
| VG | 0.67 | 0.63 | 0.10 | 0.08 | -0.15 | 0.24 | 1 | 0.33 | -0.30 |
| GW | 0.47 | 0.40 | 0.35 | 0.33 | 0.18 | 0.20 | 0.29 | 1 | -0.22 |
| PC | -0.08 | -0.06 | 0 | 0.08 | 0.08 | 0.03 | -0.20 | -0.13 | 1 |
NEPot: Number of Ears per Pot. NEPlnt: Number of Ears per Plant. EW: Average Weight of Ears. SE: Number of Spikelet’s. SS: Number of Sterile Spikelet’s. NGE: Number of Grains per Ear. VG: Number of Viable Grains. GW: Grain Weight. PC: Grain Protein Content.
Table 7. Genetic correlation matrix (above the diagonal) and phenotypic correlation matrix (below the diagonal) of the traits identified as principal components in the control environment
The joint analysis of correlations (Table 8) indicated that the phenotypic correlation for EW and NGE was superior to the genotypic. The traits VG and NEPlnt presented as genetically and phenotypically correlated in the same direction, this correlation being of medium magnitude.
| Variables | NEPot | NEPlnt | EW | SE | SS | NGE | VG | GW | PC |
|---|---|---|---|---|---|---|---|---|---|
| NEPot | - | 0.91 | 0.08 | 0.23 | 0.03 | 0.27 | 0.67 | 0.53 | -0.22 |
| NEPlnt | 0.89 | - | 0.04 | 0.19 | 0.00 | 0.23 | 0.66 | 0.48 | -0.23 |
| EW | 0.32 | 0.22 | - | 0.52 | 0.05 | 0.71 | 0.03 | 0.48 | -0.19 |
| SE | 0.18 | 0.14 | 0.51 | - | 0.72 | 0.42 | 0.12 | 0.36 | 0.03 |
| SS | -0.08 | -0.05 | -0.16 | 0.64 | - | -0.28 | -0.11 | 0.06 | 0.28 |
| NGE | 0.29 | 0.21 | 0.82 | 0.47 | -0.35 | - | 0.35 | 0.45 | -0.31 |
| VG | 0.66 | 0.59 | 0.35 | 0.14 | -0.17 | 0.38 | - | 0.38 | -0.42 |
| GW | 0.60 | 0.51 | 0.55 | 0.29 | -0.06 | 0.41 | 0.46 | - | -0.29 |
| PC | -0.34 | -0.31 | -0.40 | -0.07 | 0.23 | -0.35 | -0.42 | -0.40 | - |
NEPot: Number of Ears per Pot. NEPlnt: Number of Ears per Plant. EW: Average Weight of Ears. SE: Number of Spikelet’s. SS: Number of Sterile Spikelet’s. NGE: Number of Grains per Ear. VG: Number of Viable Grains. GW: Grain Weight. PC: Grain Protein Content.
Table 8. Genetic correlation matrix (above the diagonal) and phenotypic correlation matrix (below the diagonal) of the traits identified as principal components in both environments.
Discussion
In the principal components analysis (PCA), we can observe that the GW trait presents a high negative correlation with the principal component 1 (PC1) in both environments, showing to be the trait with greater variance, being even greater in the heat environment. We can also observe that the SS trait was retained in the CP2 in the Heat environment, while in the Control environment it was only retained in the CP3, evidencing that the Heat provokes greater variability in the spikelet sterility.
Analyzing the average of genotypes discriminated by PCA in both environments (Tables 3 and 5), the stressful environment negatively influenced almost all traits, except for the number of spikelet’s per ear (SE) and protein content (PC). However, despite the SE characteristic not being influenced by temperature, it is clear that the spikelet’s suffered from stress when we analyze the traits number of sterile spikelet’s (SS) and number of grains per ear (NGE), indicating that SE should not be considered separately as grain production parameter.
The number of viable grains (VG) was also significantly lower in the stressful environment genotypes. According to Souza et al., (2011) and Majoul et al., (2003), the high temperature implies grain yield losses, with the development of shriveled grain, reduced weight, and reduced wheat commercial quality, mainly due to damage caused during the grain filling phase (Dias et al., 2008).
The BRS Guabiju genotype (21), despite producing a large number of ears, was the most affected by infertility. The occurrence of heat during anthesis increases the sterility of flowers, affecting the number of grains per ear (Farooq et al., 2011), even causing complete sterility (Neilson et al., 2010). According to Hedhly et al., (2009), wheat plants exposed to 30°C over a period of three days during anthesis had abnormal anthers and structural and functional disability in 80% of the spikelet’s. The most critical cases of heat stress occur in the pollination, anthesis and grain filling stages. During pollination, the high temperature affects the viability of the pollen leading to the formation of non-viable seeds via floral abortion (Young et al., 2004). The Fundacep Bravo genotype (50), showed a lower number of sterile spikelet’s and although also having the lowest average for SE and PC, it produced good amount of grain considered viable.
Despite low fertility, the heat did not damage the grains protein content. According to Randall and Moss, (1990), protein accumulation has a reduced sensitivity to high temperatures than starch, however, according to Peterson et al., 1998, even without reduction in the grain protein content, in some cases, thermal stress can lead to a decrease in the derived bread volume and the sedimentation volume. The genotype PF100332 (79), although having the lowest grain weight (GW) average, contributed to an increase on protein content (PC) average among genotypes discriminated in the heat environment, by presenting a PC of 16.54%, indicating to be a potential genotype for introducing the high protein content trait in crosses with other genotypes which have lower infertility rates caused by heat.
The genotypes CD 104 (26), CD 122 (34) and TBIO Itaipú (91) presented as promising for crops in warmer environments, as they provided good averages for traits GW (26), EW and NGE (34) and VG (91). In the correlation analyses for both environments, the fact that genetic correlations between NEPlnt and NEPot traits were high and in the same direction indicates that, in a production environment where there is competition, the characteristic, NEPlnt may represent the number of ears to be produced in a specific production area. The phenotypic correlation was also high and same direction, indicating that the environment acts very little and similarly on the traits in question.
The results of phenotypic and genotypic correlation between SE and SS indicates a direct relationship between ear infertility and heat stress. According to Dolferus et al., (2011), the occurrence of high temperatures at anthesis and five days prior to it, can cause drastic reductions in wheat production, because there is a formation of pollen grains during this period, leading to their sterility. Under heat, was observed sucrose and starch depletion in tomato and bell pepper pollen (Aloni et al., 2001; Pressman et al., 2002), as well as a reduction in the activity of invertase, responsible for starch accumulation in cell wall sorghum pollen (Jain et al., 2007, 2010).
The joint analysis of correlations indicated that the phenotypic correlation for EW and NGE was superior to the genotypic, indicating that, on average, the environment tends to favor the traits.
Overall, the results indicated that the traits are good paramet ers for analysis and selection of genotypes, as they indicated they contain considerable total available variation among the genotypes studied. However, it is important that these traits be interpreted together so there is no productivity overestimation, since, as noted, the temperature did not affect the number of formed ears, but did affect the fertility of spikelet’s.
Conclusion
Heat stress causes significant losses in wheat production and leads to the formation of sterile spikelet’s. In this work, had no influence on the protein content of wheat grains. The production components (GW, NGE and VG) are those that best discriminate genotypes in the environment subjected to heat stress. Among the wheat genotypes analyzed, CD 104, CD 122 and TBIO Itaipu have the best performance when subjected to heat stress.
Acknowledgments
The authors thank the Foundation for Research Support of the State of São Paulo (FAPESP) for the financial support of this project. (Process number: 2014/21127-1).
About the Authors
Corresponding Author
AAP Correa
Department of Plant Production-Faculty of Agricultural Sciences and Veterinary Sciences/Unesp, Jaboticabal, São Paulo, Brazil
- Email:
- aretiss@yahoo.com.br
References
- Aloni B, Peet M, Pharr M, Karni L (2001). The effect of high temperature and high atmospheric CO2 on carbohydrate changes in bell pepper (Capsicum annuum) pollen in relation to its germination. Physiologia Plantarum 112: 505-512. https://doi.org/10.1034/j.1399-3054.2001.1120407.x
- CONAB - Companhia Nacional de Abastecimento. Acomp. Safra Bras. Grãos, v.4 - Safra 2016/17, Décimo Primeiro Levantamento, Brasília, p. 1-171, agosto 2017. –Disponível em:
- Condé ABT, Andrade AT, Martins FAD, Sobrinho JS, et al. (2013). Trigo de sequeiro: potencialidades. Informe Agropecuário 34: 24-29.
- Dias AS, Bagulho AS, Lidon FC (2008). Ultrastructure and biochemical traits of bread and durum wheat grains under heat stress. Brazilian Journal of Plant Physiology 20: 323-333. https://doi.org/10.1590/s1677-04202008000400008
- Dolferus R, Ji X, Richards RA (2011). Abiotic stress and control of grain number in cereals. Plant science. 181: 331-341. https://doi.org/10.1016/j.plantsci.2011.05.015
- Farooq M, Bramley H, Palta JA (2011). Heat stress in wheat during reproductive and grain-filling phases. Critical Reviews in Plant Sciences 30: 491-507.https://doi.org/10.1080/07352689.2011.615687
- Ferraudo AS (2010) Técnicas de Análise Multivariada. StatSoft South América, São Caetano, SP.
- Galvani F, Gaertner E (2006). Adequação da metodologia Kjeldahl para determinação de nitrogênio total e proteína bruta. XI MET. 34.
- Hedhly A, Hormaza JI, Herrero M (2009). Global warming and sexual plant reproduction. Trends in Plant Science 14: 30-36. https://doi.org/10.1016/j.tplants.2008.11.001
- Jain M, Chourey PS, Boote KJ, Allen Junior LH (2010). Short-term high temperature growth conditions during vegetative-to-reproductive phase transition irreversibly compromise cell wall invertase-mediated sucrose catalysis and microspore meiosis in grain sorghum (Sorghum bicolor), Journal of Plant Physiology 167: 578-582. https://doi.org/10.1016/j.jplph.2009.11.007
- Jain M, Prasad PV, Boote KJ, Hartwell Junior AL (2007). Effects of season-long high temperature growth conditions on sugar-to-starch etabolism in developing microspores of grain sorghum (Sorghum bicolor L. Moench), Planta. 227: 67-79. https://doi.org/10.1007/s00425 007-0595-y
- Kaiser HF (1958). The varimax criterion for analytic rotation in factor analysis. Psychometrika. 23: 187-200. https://doi.org/10.1007/bf02289233
- Machado JC, Souza MA, Oliveira DM (2010). Recurrent selection as breeding strategy for heat tolerance in wheat. Crop Breeding and Applied Biotechnology. 10: 9-15. https://doi.org/10.12702/1984-7033.v10n01a02
- Majoul T, Bancel E, Triboi E, Bem Hamida J (2003). Proteomic analysis of the effect of heat stress on hexaploid wheat grain: Characterization of heat-responsive proteins from total endosperm. Proteomics 3: 175-183. https://doi.org/10.1002/pmic.200390026
- Moreira RMP, Ferreira JM, Takahashi LSA, Vasconcelos MEC (2009). Potencial agronômico e divergência genética entre genótipos de feijão-vagem de crescimento determinado. Semina: Ciências Agrárias 30: 1051-1060. https://doi.org/10.5433/1679-0359.2009v30n4sup1p1051
- Neilson KA, Gammulla CG, Mirzaei M (2010). Proteomic analysis of temperature stress in plants. Proteomics. 10: 828-845. https://doi.org/10.1002/pmic.200900538
- Orr ML, Watt BK (1957). Amino acid content of foods. Washington: USDA. 46.
- Peterson CJ, Graybosch RA, Shelton DR, Baenziger PS (1998) Baking quality of hard winter wheat: response of cultivars to environments in the Great Plains. Euphytica 100: 157-162. https://doi.org/10.1007/978-94-011-4896-2_30
- Pressman E, Peet MM and Pharr DM (2002). The effect of heat stress on tomato pollen traits is associated with changes in carbohydrate concentration in the developing anthers. Annals of Botany. 90: 631-636. https://doi.org/10.1093/aob/mcf240
- Randall PJ, Moss HJ (1990). Some effects of temperature regime during grain filling on wheat quality. Australian Journal of Agricultural Research 41: 603-617. https://doi.org/10.1071/ar9900603
- Resende MDV (2006). O software Selegen Reml/Blup. Campo Grande: Embrapa Gado de Corte. 299.
- Souza MA, Pimentel AJB (2013). Estratégias de seleção para melhoramento do trigo com tolerância ao estresse por calor. IN: EMPRESA DE PESQUISA AGROPECUÁRIA DE MINAS GERAIS. Informe Agropecuário. Belo Horizonte, MG: Epamig. 34:30-39.
- Souza MA, Pimentel AJB, Ribeiro G (2011). Melhoramento para tolerância ao calor. Melhoramento de plantas para condições de estresses abióticos. Visconde do Rio Branco: Suprema, cap.9. p.199-226.
- Souza MA, Ramalho MAP (2001). Controle genético e tolerância ao estresse de calor em populações híbridas e em cultivares de trigo. Pesquisa Agropecuária Brasileira, Brasília, v.36, n.10, p.1245-1253. https://doi.org/10.1590/s0100-204x2001001000005
- Statsoft, Inc. (2010). Statistica, versão 10. www.statsoft.com
- Young LW, Wilen RW, Bonham-Smith PC (2004). High temperature stress of Brassica napus during flowering reduces micro- and megagametophyte fertility, induces fruit abortion, and disrupts seed production. Journal of Experimental Botany. 55:485-495. https://doi.org/10.1093/jxb/erh038
Keywords:
Download:
Full PDF- Share This