Intestinal Dysbiosis Increases the Incidence of Malignant Melanoma in Mice Model
Received: September 11, 2017
Accepted: November 08, 2017
Published: November 29, 2017
Genet.Mol.Res. 16(4): gmr16039840
DOI: 10.4238/gmr16039840
Abstract
Antibiotic-induced disruption of the intestinal microbiota has serious consequences for human physiology. We conducted a microbial dysbiosis animal model to study and directly provide evidence for microbial dysbiosis contribution to malignant melanoma animal model. Females C57BL/6 wild-type (WT) mice injected with a limiting threshold number of B16/F1 melanoma cells and the tumor development was observed with and without probiotics treatment. Treatment with probiotic could restore microbiota diversity and indirectly effect the tumor incidence. In microbial dysbiosis group tumor incidence was 83.33%. Also, the expression level of inflammatory proteins NFkB-p65; IL-6; and STAT-3 were remarkably enhanced in microbial dysbiosis group compared to probiotic treated group. Altogether, our findings demonstrated that microbial dysbiosis can strike the balance between immunity and tumorigenesis, and increase the incidence of malignant melanoma in mice. Probiotic treatment significantly reduced tumor incidence
Introduction
Antibiotics are one of the greatest inventions of the world, it is difficult to imagine the life without antibiotics. Yet, every coin has two sides, antibiotic exposures may increase the risks of metabolic and neoplastic disease by disrupting the microbiomes (Blaser MJ, 2016). Dysregulated microbiota lead to a variety of diseases, such as obesity-related disease, liver disease, IBD and colorectal cancer (Marchesi JR, et al., Fukuda S, Hara E, Yamamoto M, Matijasic M, 2016) Melanoma is one of the most aggressive types of skin cancer (Miller AJ, 2006) and the average life expectancy after diagnosis was dismal (Koller KM, 2016) Malignant cells can evade the host immune system (Hude I, et al. 2017) As reported in our previous study, we conducted an animal model which can sensitively detect the changes of immune level (Owusu L, 2015). Briefly, animals were injected with B16/F1 melanoma cells in the limiting threshold number of 1×103 cells. In normal immune state, animals will not get tumor with the mentioned threshold, if the immune state is low, the tumor will breakout. Based on this method, we conducted a microbial dysbiosis model using antibiotics, and injected animals with B16/F1 melanoma cells in the limiting threshold of 1× 103.
The notion that inflammation drives cancer is already well established. The inflammatory gene nuclear factor kappa B (NF-kB) has been found constitutive activity in most solid and lymphoid tumours (Grivennikov SI, 2010) Interleukin-6(IL-6) has been reported as a prognostic factor in melanoma. Enhanced levels of IL-6 are predictive for a high tumor burden and a reduced overall survival in metastatic melanoma patients (Soubrane C, 2005) There exists an NF-kB/IL-6/STAT-3 cascade involved in tumorigenesis, which IL-6 acts as an important component.
This work focused on the effects of microbial dysbiosis and probiotics on the incidence of malignant melanoma through PCR-DGGE, tumorigenesis and the identification of biomolecular markers of inflammation.
Materials and Methods
Animals and cell line
Six weeks old female C57BL/6 wild-type (WT) mice were obtained from SPF (Specific Pathogenic Free animals Centre) of Dalian Medical University (Liaoning, China). B16/F1 melanoma cells were purchased from (ATCC, number: CRL-6323, USA) were performed in accordance with protocols described before. The animal experiments were approved by Dalian Medical University Animal Care and Use Committee.
Probiotic preparation
LBB was obtained from Biostime Inc (Guangzhou, China). The formula of LBB was Lactobacillus acidophilus, Bifidobacteria spp (B. bifidum and B. infantum) and Fructo-oligosaccharide and maltodextrin as supplement. The dosage of the probiotic formula was calculated according to Meeh-Rubner conversion formula between human and mice (Xinli L, 2013). The probiotic formula was administered orally at 0.9 g/kg body weight daily after conversion.
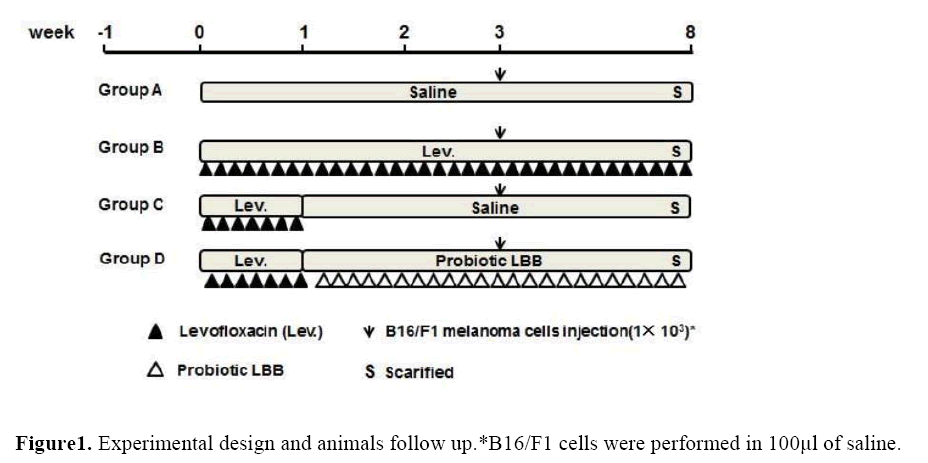
Experimental design and animals follow up
After one week of acclimatization, animals were divided into 4 groups, each group were 6 animals. They were treated as follows (See Figure 1):
Group A (melanoma cells only): animals were oral gavaged with saline as control, then received B16/F1 melanoma cells injection with the limiting threshold number 1× 103 in the third week.
Group B (antibiotic + melanoma cells): animals were oral gavaged with daily dose of Levofloxacin from the beginning to the end of the experiment, and received B16/F1 melanoma cells injection with the limiting threshold number 1× 103 in the third week.
Group C (antibiotic + self-recover + melanoma cells): animals were oral gavaged with daily dose of Levofloxacin for one week, then self-recover to the end of the experiment, and received B16/F1 melanoma cells injection with the limiting threshold number 1× 103 in the third week.
Group D (antibiotic + probiotics + melanoma cells): animals were oral gavaged with daily dose of Levofloxacin for one week, and oral gavaged with probiotics from the second week to the end of the experiment. In the third week the animals received B16/F1 melanoma cells injection with the limiting threshold number of 1× 103.
Intestinal microbiota analysis
Fresh stool samples were placed in sterile centrifuge tubes and stored at -80Ã?Â?C prior to use. Genomic DNA was extracted from stool samples using Power Soil DNA Isolation Kit (MO BIO) according to the manufacturer’s instruction. Genomic DNA was amplified with universal bacterial primers which was described before (Liu J, et al, 2012)
PCR amplification was performed in an automated thermocycler (Thermo, USA). The quality of PCR products was tested by electrophoresis on 1.0% (w/v) agarose gel. The method of DGGE (Denaturing Gradient Gel Electrophoresis) was according to the work previously reported (Liu J, 2015). Subsequently, the gels were washed with ultrapure water and stained with 5% EB (ethidium bromide) for 45 mins and photographed. DGGE graphs were digitized by Phoretix 1D software (Phoretix, Newcastle upon Tyne, UK).
After this, DGGE bands were selected and analyzed. Bands were cut with a sterile surgical blade under UV, washed, and kept in sterile centrifuge tubes with 100μL sterile water at 4°C overnight. Then put the tube into 90°C water bath for 10 minutes, and the solution was used as DNA template, original primers without GC clamp for PCR. PCR products were ligated into the pMD18-T vector and transformed into competent E. Coli Nova Blue (TaKaRa, Japan). The recombinant plasmids were isolated and sequenced by company (TaKaRa, Japan). Then the sequences were aligned to the NCBI by BLAST search.
B16/F1 melanoma cells transplant model
C57BL/6 WT mice received subcutaneous implantation of B16/F1 melanoma cells with the limiting threshold of 1× 103 at the flank. B16/F1 cells were performed in 100μl of saline. Animals were monitored for tumor growth at 2-day intervals for at least 45 days, and up to 90 days for non-tumor bearing mice. In a tumor bearing animal, tumor maximal width, and maximal perpendicular length were measured with a digital calliper. Also, the number of tumor was calculated in different groups.
Hematoxylin and Eosin(HE) staining of colon tissue
Animals were sacrificed, and colons were collected and placed in 10.0% formaldehyde, rested overnight. Hematoxylin and eosin (HE) staining was carried out in Histopathology Laboratory at the Dalian Medical University and read by a pathologist who was blinded to the experimental design.
Immunohistochemistry(IHC) analysis of colon tissue
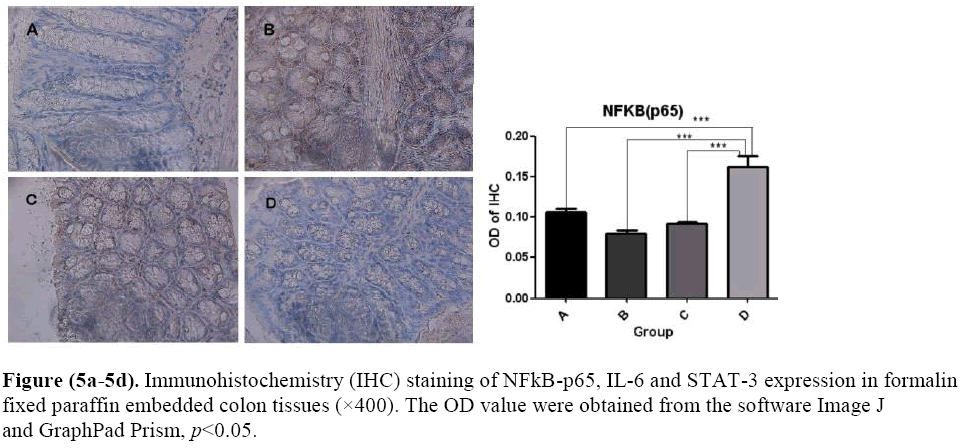
Paraffin-embedded sections of colon tissue were made by the Histopathology Laboratory at the Dalian Medical University. Sections were deparaffinized in xylene, hydrated through a series of graded ethanol solutions, and subjected to antigen retrieval in 10mM citrate buffer (pH 6.0) in microwave oven for 20 minutes. The endogenous peroxidase activity was blocked, and DAB were done using Splink detection kit (ZSGB-BIO, Beijing) according to manufacturer’s protocol. Sections were incubated in 4°C with NFkB-p65 antibody (1:100 dilution) for 16 hours. NFkB-p65was expressed as mean intensity of NFkB-p65 staining, calculated as ratio of mean area of NFkB-p65 staining and mean integrated optical density using the Image J software.
Statistical analysis
Data were analyzed with GraphPad Prism software (version 6.04, USA). ANOVA followed by Turkey’s post hoc analysis was used to analyze differences in microbiota abundance at various levels. Significant differences between groups were assessed by Student's t test. Fisher’s exact test was employed to analyze the difference in tumor incidence. Results were considered significant at p < 0.05.
Results
Antibiotic treatment induced microbial dysbiosis.
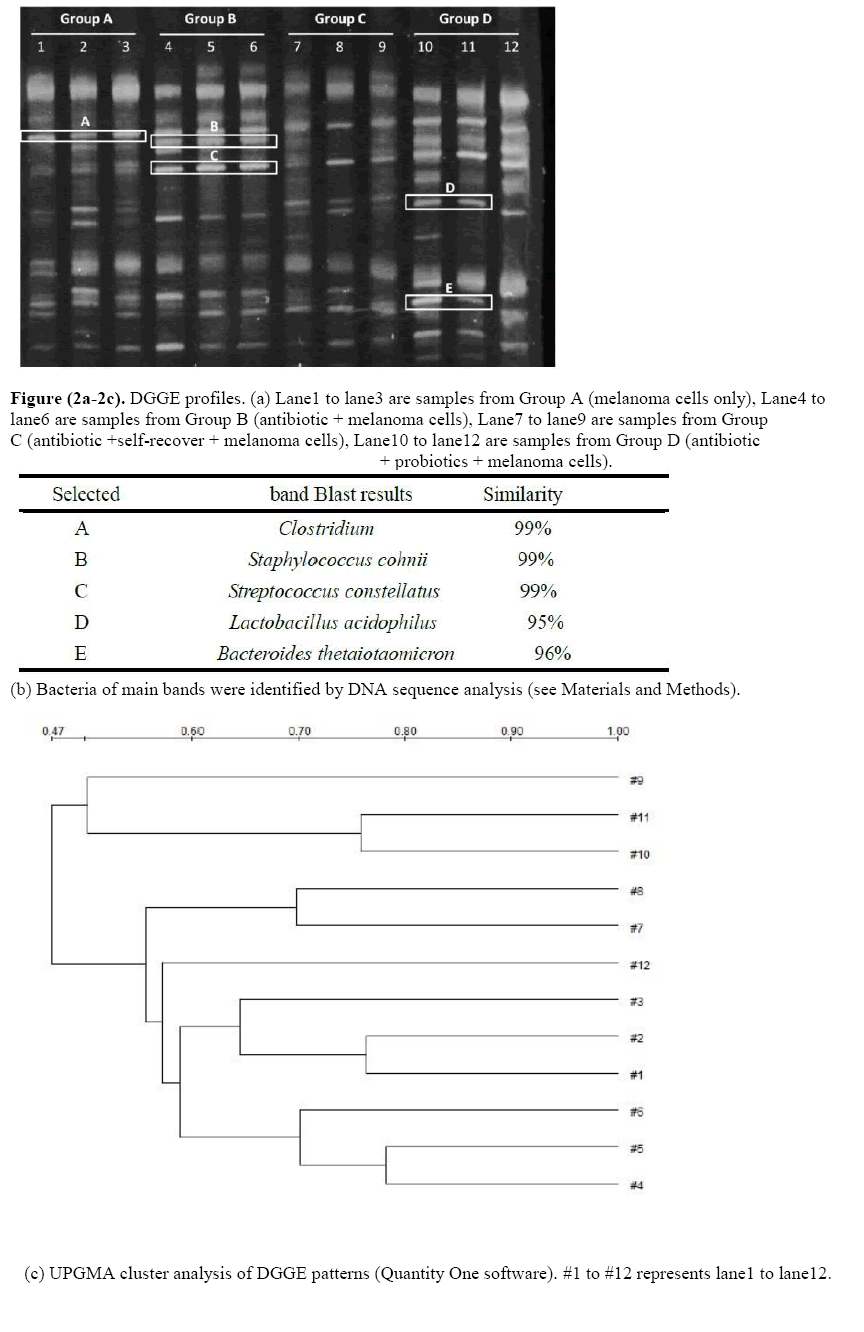
The DGGE images of the 12 faecal samples were obtained by applying PCR-DGGE technology (Figure 2a). Lane1 to lane3 are Group A (melanoma cells only), lane 4 to lane 6 are Group B (antibiotic + melanoma cells), lane 7 to lane 9 are Group C (antibiotic +self-recover + melanoma cells), lane 10 to lane 12 are Group D (antibiotic + probiotics + melanoma cells). A decreased gut microbial diversity was shown in Group B and Group C. The UPGMA cluster analysis of DGGE patterns by Quantity One software showed that the 12 examined samples were distinctly separated into different clusters (Figure 2c). Samples from Group A and Group B were in their own clusters, sample 9 which has farthest clustering distance with sample7 and 8 from the same group. The results indicated that after antibiotic treated, the self-recover ability of individuals was different from each other, the probiotic treated can restore the diversity of microbiota. Also, the sequencing results of the selected bands both matched to BLAST results (Figure 2b).
Figure (2a-2c): DGGE profiles. (a) Lane1 to lane3 are samples from Group A (melanoma cells only), Lane4 to lane6 are samples from Group B (antibiotic + melanoma cells), Lane7 to lane9 are samples from Group C (antibiotic +self-recover + melanoma cells), Lane10 to lane12 are samples from Group D (antibiotic + probiotics + melanoma cells).
Microbial dysbiosis increased the tumorigenesis in animal model.
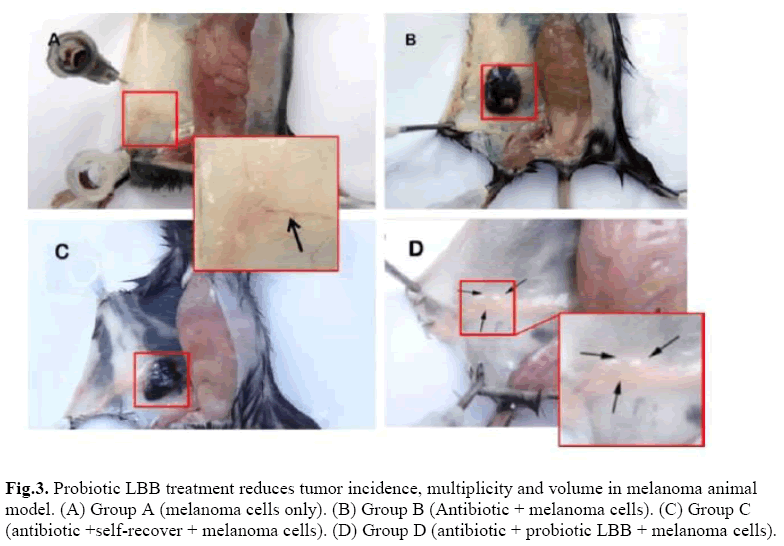
The effect of microbial dysbiosis on immunity was studied in melanoma animal model (Figure 3). At the end of the experiment, tumor incidence in Group B was 83.33%, in Group C was 33.33% (p <0.05). Angiogenesis were found in Group A, without solid tumor, as the arrow shows (Figure 3A). The tumor size was biggest in Group B compared to the Group C (Figure 3.BC). However, no angiogenesis was found in Group D and the injected melanoma cells were restricted into a small ball (Figure 3D).
Figure 3: Probiotic LBB treatment reduces tumor incidence, multiplicity and volume in melanoma animal model. (A) Group A (melanoma cells only). (B) Group B (Antibiotic + melanoma cells). (C) Group C (antibiotic +self-recover + melanoma cells). (D) Group D (antibiotic + probiotic LBB + melanoma cells).
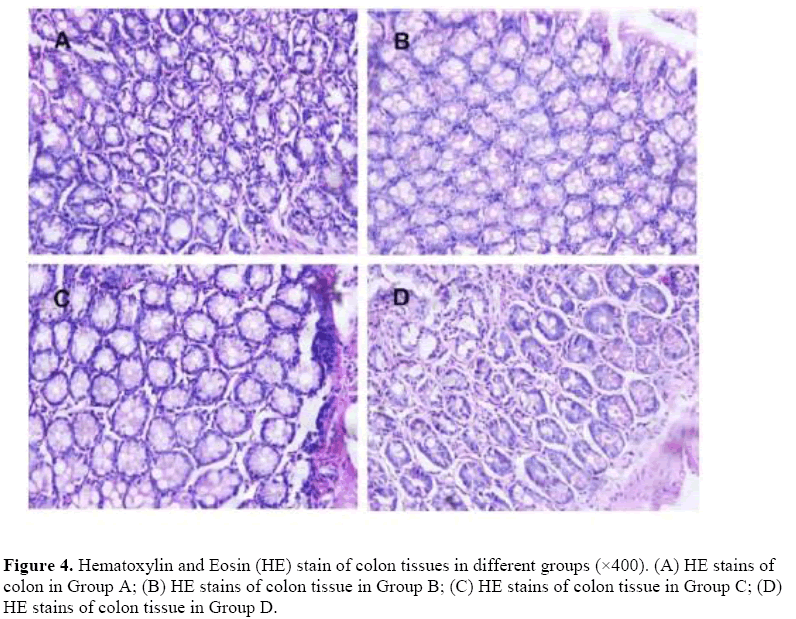
Microbial dysbiosis didn’t change the physical structures of colon.
We used the Hematoxylin and Eosin (HE) stain method to the see whether the dysregulated microbiota can change the physical structures of colon. The HE is staining of colon tissues from each group, shown that the nucleus structure is normal, no abnormal morphological changes were found in each group (Figure 4).
Microbial dysbiosis trigger the inflammation in tumor progression.
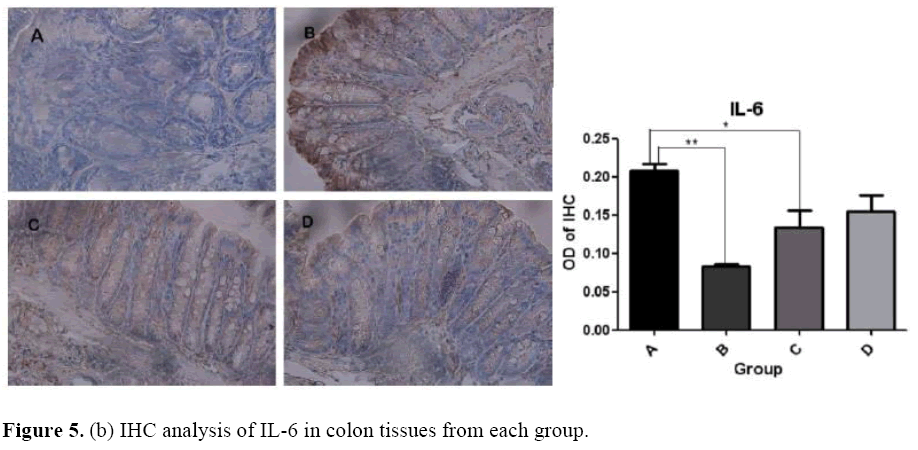
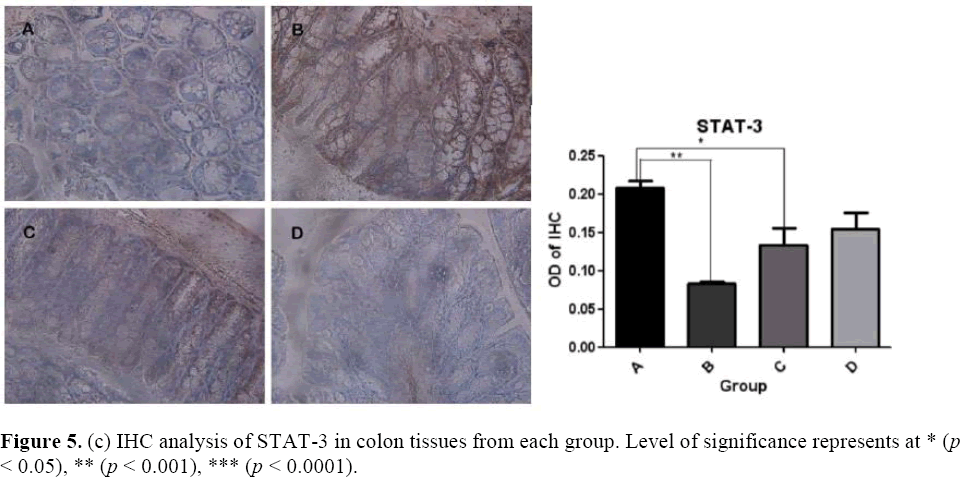
The effects of microbial dysbiosis on the inflammatory cytokines NFkB-p65, IL-6 and STAT-3 were investigated by IHC. We observed an increased expression level of NFkB-p65, IL-6 and STAT-3 in Group B but decreased in Group D (Figure 5). Also, we got the OD value from the IHC results by using the Image J and GraphPad Prism software. The OD value is contrast to the expression level. If the OD value is high, means that the expression level of the protein is low.
Discussion
Microbial dysbiosis, has been implicated in various human diseases including obesity (Ohtani N, 2014) diabetes (He C, 2015), colon cancer (Belcheva A, 2015) and breast cancer (Xuan C, 2014 et al.). In our study, we investigated the effects of microbial dysbiosis on the progression of malignant melanoma in animal model, which give a powerful evidence for the relationship between microbial dysbiosis and cancer. Also, the present study demonstrated that probiotics can reduce the occurrence of tumor by anti-inflammation. The use of antibiotic can induce imbalances in gut microbiota, called dysbiosis (Vangay P, 2015) which is reflected in this study by PCR-DGGE. A decreased gut microbial diversity was observed after antibiotic treated. The sequencing results showed that, the Band C is Streptococcus constellatus, which is often involved in suppurative infection at various body sites, especially in patients with malignancy and immunodeficiency (Ko T, et al.) Band E is Bacteroides thetaiotaomicron, an important member of the host’s normal flora, which is symbiont pivotal for host immunity, by producing an immunostimulatory LPS comprised of penta-acylated lipid A. Penta-acylated lipid A has been observed to greatly influence the innate immune response of the host (Berezow AB, et al., 2009). Band D is Lactobacillus acidophilus (L. a), a member of probiotics, which have been shown can beneficial hosts on various ailments including malignancies. L. a can activate NK cells and act as an active participant in anti-tumor immunity (Maroof H, Wen K, et al. A great density of Band D and Band E was observed in Group D. Tumor incidence in Group B was 83.33%, Group C was 33.33%, but in control group (Group A) and probiotics rescue group (Group D) was 0.00%. This observation confirmed that probiotics were able to suppress tumorigenesis. Indicating that, the host immunity was enhanced after probiotic treated.
The formation of new blood vessels, called angiogenesis, is essential for tumor growth (Rittling SR, 2014) Tumor angiogenesis were found in Group A, B, C, but no angiogenesis was found in Group D. Studies have shown that metastatic melanoma cells constitutively express a high level of NF-kB activity, which can increase angiogenesis (Huang S, 2000). NF-kB plays a major role in linking inflammation to cancer development through its ability to up regulate inflammatory and tumor promoting cytokines like: IL-6, IL-1 α and TNF α, as well as genes like BCL2 and BCLXL (Jurjus A, et al. 2016). Aberrant NF-kB regulation has been observed in many cancers, whose sustained activation requires STAT-3. STAT3 shown to be aberrantly activated in multiple solid and liquid tumours, which promotes tumorigenesis by regulating the expression of various target genes, including angiogenic factors and anti-apoptosis genes (Wake MS, 2015). STAT3 activation may induce expression of inflammatory cytokines such as IL–6 and angiogenic factors such as HIF-1a and VEGF (Xu Q, Ogura H 2008) IL-6 facilitates tumor growth primarily by inhibiting apoptosis and enhancing cell proliferation (Kumari N, 2016). The activation of IL-6/STAT-3 signalling axis can promote tumorigenesis by regulating multiple survival signalling pathways in cancer cells (Mihara M, 2012). IL-6/STAT-3 signalling is also required for protecting normal and pre-malignant epithelial cells from cell in colitis-associated cancer (Grivennikov S, et al. 2009). These signalling pathways help tumours in the acquisition of unlimited replication potential, which is essentially required to generate large tumours. Since NF-kB and STAT-3 are regulated by IL-6, it appears that the NF-kB/STAT-3/IL-6 signalling cascade plays an important role in oncogenesis.
Studies have shown that probiotic LBB were able to express anti-inflammatory cytokines, the immunomodulation activity of probiotics was proposed in different disease (Fang H, et al. 2000). The results obtained in the present study suggest that the immune benefits of probiotic LBB may be partly attributed to down regulated NF-kB, IL-6 and STAT-3. Hence, the microbial dysbiosis trigger the inflammation in tumor progression. While, probiotic LBB can suppresses them, which associated with cancer prevention.
To the best of our knowledge, it is the first time to detect the influence of microbial dysbiosis on immune system by limiting threshold method, which may contribute to future studies on microbial dysbiosis and disease. The use of probiotics can contribute to adjuvant therapies strategies to reduce the risk of tumor occurrence.
Conclusion
Our data further suggests that antibiotic can weaken the immunity of the host through disturb the microbiota. The injection of the limiting threshold number of malignant melanoma cells can accurately indicate the changes of the immune level. Probiotics contribute to restore the microbiota diversity thus reduce the risk of tumor occurrence.
Funding
The study was kindly supported by the National Natural Science Foundation of China (81373875).
Conflicts of interest
The authors declare that they have no competing interests.
ETHICAL APPROVAL
This work followed the ethical guidelines and was approved by the Dalian Medical University Animal Care and Use Committee.
About the Authors
Corresponding Author
Yi Xin
Department of Biotechnology, Dalian Medical University, Dalian, 116011, China
- Email:
- jimxin@hotmail.com
References
- Blaser MJ (2016). Antibiotic use and its consequences for the normal microbiome. Science 352 (6285):544-545.https://doi.org/10.1126/science.aad9358
- Marchesi JR, Adams DH, Fava F, Hermes GDA, et al. (2016). The gut microbiota and host health: a new clinical frontier. Gut 65 (2):330-339. https://doi.org/10.1136/gutjnl-2015-309990
- Fukuda S, Ohno H (2014). Gut microbiome and metabolic diseases. Seminars in immunopathology. 36 (1):103-114. https://doi.org/10.1007/s00281-013-0399-z
- Hara E (2015). Relationship between Obesity, Gut Microbiome, and Hepatocellular Carcinoma Development. Digestive diseases 33 (3):346-350. https://doi.org/10.1159/000371679
- Yamamoto M, Matsumoto S (2016). Gut microbiota and colorectal cancer. Genes and environment: the official journal of the Japanese Environmental Mutagen Society. 38:11.
- Matijasic M, Mestrovic T, Peric M, Cipcic Paljetak H, et al. (2016). Modulating Composition and Metabolic Activity of the Gut Microbiota in IBD Patients. International journal of molecular sciences 17 (4). https://doi.org/10.3390/ijms17040578
- Miller AJ, Mihm MC Jr. (2006) Melanoma. The New England journal of medicine 355 (1):51-65
- Koller KM, Wang W, Schell TD, Cozza EM, et al. (2016). Malignant melanoma-The cradle of anti-neoplastic immunotherapy. Critical Reviews in Oncology/Hematology. 106:25-54.https://doi.org/10.1016/j.critrevonc.2016.04.010
- Hude I, Sasse S, Engert A, Brockelmann PJ (2017). The emerging role of immune checkpoint inhibition in malignant lymphoma. Haematologica. 102 (1):30-42. https://doi.org/10.3324/haematol.2016.150656
- Owusu L, Wang B, Du Y, Li W, et al. (2015). The Quantum of Initial Transformed Cells Potentially Modulates the Type of Local Inflammation Mechanism Elicited by Surrounding Normal Epithelial Tissues and Systemic Immune Pattern for Tumor Arrest or Progression. Journal of Cancer. 6 (2):128-138.https://doi.org/10.7150/jca.10787
- Grivennikov SI, Karin M (2010). Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine & growth factor reviews 21 (1):11-19.https://doi.org/10.1016/j.cytogfr.2009.11.005
- Soubrane C, Rixe O, Meric JB, Khayat D, et al. (2005).Pre-treatment serum interleukin-6 concentration as a prognostic factor of overall survival in metastatic malignant melanoma patients treated with bio chemotherapy: a retrospective study. Melanoma research. 15 (3):199-204. https://doi.org/10.1097/00008390-200506000-00009
- Xinli L, Dachang W, Cuili Z, Yi X, et al. (2013). Side effects of antibiotics on the intestinal microflora by PCR-DGGE. Pakistan journal of pharmaceutical sciences 26 (2):339-343
- Liu J, Wu D, Ahmed A, Li X, et al. (2012). Comparison of the gut microbe profiles and numbers between patients with liver cirrhosis and healthy individuals. Current microbiology 65 (1):7-13. https://doi.org/10.1007/s00284-012-0105-8
- Liu J, Yan Q, Luo F, Shang D, et al. (2015). Acute cholecystitis associated with infection of Enterobacteriaceae from gut microbiota. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 21 (9):851 e851-859.https://doi.org/10.1016/j.cmi.2015.05.017
- Ohtani N, Yoshimoto S, Hara E (2014). Obesity and cancer: a gut microbial connection. Cancer research 74 (7):1885-1889. https://doi.org/10.1158/0008-5472.can-13-3501
- He C, Shan Y, Song W (2015) Targeting gut microbiota as a possible therapy for diabetes. Nutrition research 35 (5):361-367. https://doi.org/10.1016/j.nutres.2015.03.002
- Belcheva A, Irrazabal T, Martin A (2015). Gut microbial metabolism and colon cancer: can manipulations of the microbiota be useful in the management of gastrointestinal health? Bioassay: news and reviews in molecular, cellular, and developmental biology 37 (4):403-412
- Xuan C, Shamonki JM, Chung A, Dinome ML, et al. (2014). Microbial dysbiosis is associated with human breast cancer. PloS one. 9 (1):e83744. https://doi.org/10.1371/journal.pone.0083744
- Vangay P, Ward T, Gerber JS, Knights D (2015). Antibiotics, pediatric dysbiosis, and disease. Cell host & microbe 17 (5):553-564. https://doi.org/10.1016/j.chom.2015.04.006
- Ko T, Mahara K, Ota M, Kato Y, et al. (2013). A case of prosthetic valve endocarditis caused by Streptococcus constellatus as a rare agent of endocarditis. Heart & lung: the journal of critical care 42 (5):379-381.https://doi.org/10.1016/j.hrtlng.2013.05.006
- Berezow AB, Ernst RK, Coats SR, Braham PH, et al. (2009). The structurally similar, penta-acylated lipopolysaccharides of Porphyromonas gingivalis and Bacteroides elicit strikingly different innate immune responses. Microbial pathogenesis. 47(2):68-77.https://doi.org/10.1016/j.micpath.2009.04.015
- Maroof H, Hassan ZM, Mobarez AM, Mohamadabadi MA (2012) Lactobacillus acidophilus could modulate the immune response against breast cancer in murine model. Journal of clinical immunology. 32 (6):1353-1359.https://doi.org/10.1007/s10875-012-9708-x
- Wen K, Li G, Bui T, Liu F, et al. (2012) High dose and low dose Lactobacillus acidophilus exerted differential immune modulating effects on T cell immune responses induced by an oral human rotavirus vaccine in gnotobiotic pigs. Vaccine 30 (6):1198-1207. https://doi.org/10.1016/j.vaccine.2011.11.107
- Rittling SR, Wejse PL, Yagiz K, Warot GA, et al. (2014) Suppression of tumour growth by orally administered osteopontin is accompanied by alterations in tumour blood vessels. British journal of cancer. 110 (5):1269-1277.https://doi.org/10.1038/bjc.2014.10
- Huang S, DeGuzman A, Bucana CD, Fidler IJ (2000) Nuclear factor-kappaB activity correlates with growth, angiogenesis, and metastasis of human melanoma cells in nude mice. Clinical cancer research: an official journal of the American Association for Cancer Research. 6 (6):2573-2581.
- Jurjus A, Eid A, Al Kattar S, Zeenny MN, et al. (2016). Inflammatory bowel disease, colorectal cancer, and type 2 diabetes mellitus: The links. BBA clinical 5:16-24. https://doi.org/10.1016/j.bbacli.2015.11.002
- Wake MS, Watson CJ (2015) STAT3 the oncogene - still eluding therapy? The FEBS journal 282 (14):2600-2611
- Xu Q, Briggs J, Park S, Niu G, et al. (2005). Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 24 (36):5552-5560.https://doi.org/10.1038/sj.onc.1208719
- Ogura H, Murakami M, Okuyama Y, Tsuruoka M, et al. (2008). Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. 29 (4):628-636.https://doi.org/10.1016/j.immuni.2008.07.018
- Kumari N, Dwarakanath BS, Das A, Bhatt AN (2016). Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 37 (9):11553-11572.
- Mihara M, Hashizume M, Yoshida H, Suzuki M, et al. (2012) IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clinical science. 122 (4):143-159.https://doi.org/10.1042/cs20110340
- Grivennikov S, Karin E, Terzic J, Mucida D, et al. (2009). IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer cell. 15 (2):103-113.https://doi.org/10.1016/j.ccr.2009.02.003
- Fang H, Elina T, Heikki A, Seppo S (2000). Modulation of humoral immune response through probiotic intake. FEMS immunology and medical microbiology. 29 (1):47-52.https://doi.org/10.1016/s0928-8244(00)00187-5
Keywords:
Download:
Full PDF- Share This