Lentinus crinitus strains respond differently to cultivation pH and temperature
Received: January 14, 2018
Accepted: February 24, 2018
Published: February 27, 2018
Genet.Mol.Res. 17(1): gmr16039885
DOI: 10.4238/gmr16039885
Abstract
Fungus ability to respond to environmental changes allows their adaptation to a wide variety of conditions such as pH, temperature, light, nutrient availability, chemicals, and competition among species. Temperature and pH can affect fungal growth as well as their metabolism. Knowing these factors on fungus species is relevant for the development of biotechnological applications and process optimization to produce biomass and enzymes. This study aimed to evaluate Lentinus crinitus mycelial growth and laccase production at different initial pH and temperatures of the cultivation medium. L. crinitus U15-9 reduced laccase production with pH increase with maximum production at pH 6 (39 U/mL) whereas L. crinitus U9-1 increased laccase production with pH increase with maximum activity at pH 7 (25.8 U/mL). Neither of the strains had mycelial growth significantly affected by initial pH in the culture medium. The greatest laccase production occurred at 28 ºC for U15-9 (50 U/mL) and U9-1 (41.5 U/mL). An increase in U9-1 mycelial biomass occurred when the temperature went up from 25 to 28 ºC whereas for U15-9 it was from 28 to 37 ºC; at 37 ºC, U15-9 produced 225% more mycelial biomass than at 28 ºC, and 108% more mycelial biomass than U9-1 at 37 ºC. The variation of responses to environmental stimuli between the strains makes evident that intra-specific variations are common in basidiomycetes. Our findings highlighted the best conditions of pH and temperature to produce L. crinitus laccase and make evident how different strains respond distinctly to cultivation conditions.
Introduction
Fungus development, growth, and metabolism are subject to alterations as a response to the environment. This capability allows them to adapt to a great range of conditions such as varying pH or temperature, light, nutrient availability, chemicals, competition among species, and other factors (Wu et al. 2016).
One of the main factors that affect fungus growth and physiology is pH. Fungi are usually limited to grow in a narrow pH range close to neutrality, although some can tolerate extreme pH. The environmental pH can directly affect the cell membrane and the ion dissociation degree, interfering in the absorption of nutrients and minerals (Deacon 2006). On the other hand, the secretion of compounds such as organic acids allows that fungi change the pH around them (Cervantes-Chávez et al. 2010). Adaptation to different pH requires an internal pH homeostatic system and a specific regulatory system which assures that molecules exposed to the environment be secreted only under favorable conditions (Peñalva et al. 2008). Temperature has an important role in the fungus distribution and adaptation. The environmental temperature affects the fungus enzymatic activity, causing alterations in the metabolism and growth (Chang and Miles, 1997). Therefore, it is relevant knowing how the cultivation temperature can help to establish conditions that favour biomass production and biotechnological products.
Lentinus crinitus (L.) Fr. (Basidiomycota) is a wild fungus with pantropical and neotropical distribution (Silva and Gibertoni, 2006), mainly in Brazil (Groposo and Loguercio-Leite, 2005; Gomes-Silva and Gibertoni, 2009). It is a saprophyte fungus that grows in trunks of decomposing trees (Abraham and Abate, 1995) and produce enzymes like laccase (Valle et al. 2014), cellulase, xylanase (Cambri et al. 2016), phytase (Pereira, 2015) and proteases (Kirsch et al. 2013; Machado et al. 2016) with potential applications in industrial and biotechnological processes. It also presents antioxidant activity (Umeo et al. 2015) and metal bioaccumulation capacity (Meniqueti, 2016).
Laccases (Benzenediol)
oxygen oxidoreductases, EC 1.10.3.2) are multicopper oxidases that catalyze oxidation of a great variety of phenols and polyphenols, utilizing molecular oxygen as an electron acceptor. However, there were no studies found about the effect of abiotic factors on L. crinitus mycelial growth and laccase production. In this study, the mycelial growth and laccase production of L. crinitus at different initial pH and temperatures of the cultivation medium were evaluated. Knowledge on how these abiotic factors affect this fungus is relevant for the development of biotechnological applications and process optimizations for the production of biomass and enzymes.
Material and Methods
Microorganism and inoculum
Two strains of L. crinitus (U9-1 and U15-9) belonging to the culture collection of the Graduate Program of Biotechnology Applied to Agriculture of Paranaense University were utilized. The identification of strains was done by sequencing the internal transcribed spacers (ITS) of ribosomal DNA (Valle et al., 2015). The DNA was sequenced using ABI Prism 3100 Genetic Analyzer (Applied Biosystems, Carlsbad, CA, USA). ITS sequences were compared with other sequences found in the GenBank database (http://www.ncbi.nlm.nih.gov/genbank/) by using BLAST (http://blast.ncbi.nlm.nih.gov/). The GenBank accession numbers are MG211674 (L. crinitus U9-1) and MG211675 (L. crinitus U15-9). The strains were cultivated for seven days in malt extract agar (MEA, 20 g/L) at 28°C in the absence of light and were used as inoculum for mycelial biomass and laccase production.
Effect of pH and temperature on mycelial biomass and laccase production
The strains were cultivated in conical flasks (250 mL) containing 100 mL of malt extract (ME). The culture media were autoclaved (121°C for 20 min) and after cooling the pH was adjusted to 5, 6 or 7 by adding HCl (1 M) or NaOH (1 M), previously filtered (Millipore filter with 0.22 μm). The culture media were inoculated with 3 disks of MEA containing mycelia. To analyze the effect of the cultivation temperature, on laccase production, or mycelial biomass production, the strains were cultivated in ME at different temperatures: 22, 25, 28, or 37 °C. All the cultivations were carried out for 15 days in the absence of light and the laccase production was determined on the 15th day (Valle et al., 2014). The produced mycelium was recovered by centrifugation (8000 x g, at 4°C for 10 min) and kept in an air stove at 60 °C until constant mass to determine mycelial biomass. The assays had an entirely randomized casual design and were done in triplicate. The results were submitted to analysis of variance (ANOVA) and the significant differences among the arithmetical averages were determined by Scott-Knott test (P ≤ 0.05).
Laccase assay
Laccase production was evaluated using 1 mM ABTS (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonate) as substrate. The enzymatic activity was determined in a reaction containing 200 μL cultivation medium, 700 μL water, 450 μL sodium acetate buffer (0.1 M, pH 5.0) and 150 μL ABTS (Valle et al., 2015). The reaction mixture was maintained at 30°C for 10 min, and 100 μL trichloroacetic acid solution (50 g/L) was added to the reaction to interrupt enzymatic activity. ABTS oxidation was followed by an increase in absorbance at 420 nm (Σ=36,000 M-1·cm-1). Two reaction mixtures were used as analytical control, one without the enzymatic extract, and another without ABTS. Laccase production was expressed in international units (U) and was defined as the amount of enzyme required to oxidize 1 μmol ABTS per minute.
Results
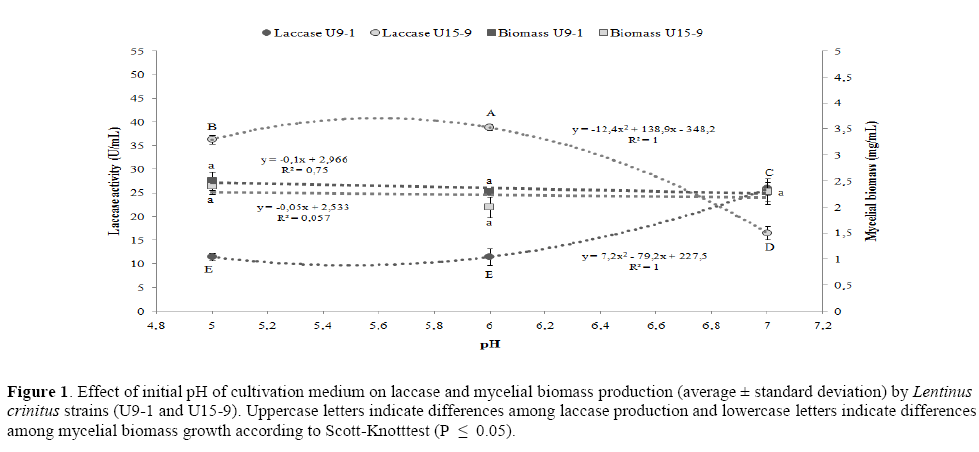
The initial pH of the cultivation medium affected laccase production quadratically with an inverse function (Figure 1). For U15-9, there was a reduction of laccase production with pH increase, shown by the negative value of α (-12.4) for the regression curve. However, for U9-1 there was an increase of laccase production with pH increase, shown by the positive value of α (+7.2) in the parabola. The greatest laccase activity was at pH 6 for U15-9 with 39 U/mL, while U9-1 produced 71% less laccase (Figure 1). On the other hand, with pH increase from 6 to 7, U15-9 laccase production reduced 57% whereas U9-1 increased 124%. The maximum theoretical enzymatic activity, obtained by the quadratic model for 15-9 strain, was 40.8 U/mL and optimal pH of 5.6; and for U9-1 strain was 25.9 U/mL with optimal pH of 7.
Figure 1: Effect of initial pH of cultivation medium on laccase and mycelial biomass production (average ± standard deviation) by Lentinus crinitus strains (U9-1 and U15-9). Uppercase letters indicate differences among laccase production and lowercase letters indicate differences among mycelial biomass growth according to Scott-Knotttest (P ≤ 0.05).
The initial pH of the cultivation medium affected the mycelial biomass production linearly (Figure 1) with little negative slope values of -0.1 for U9-1 and -0.05 for U15-9. Thus, the variation in the laccase production, in function of initial pH, is not related to the mycelial biomass production but probably due to other metabolic alterations.
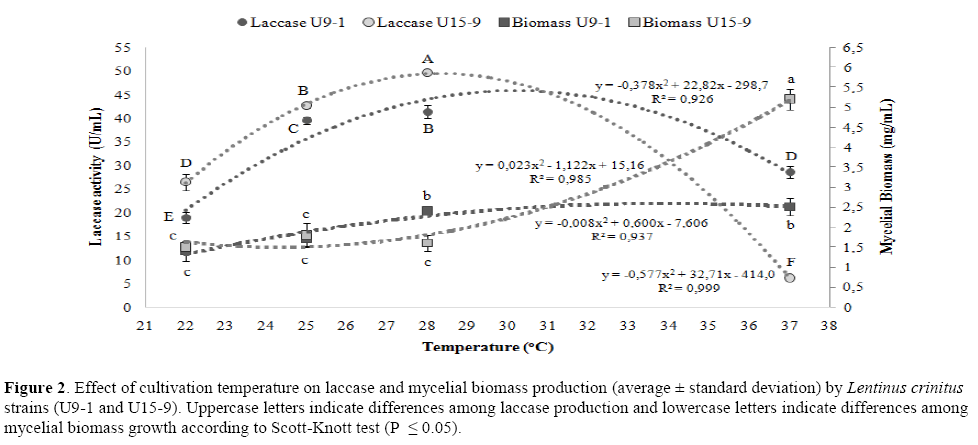
The cultivation temperature also affected laccase production quadratically; however, the polynomial function was similar for all strains (Figure 2). The greatest laccase production occurred at 28°C for U15-9 (50.0 U/mL) and U9-1 (41.5 U/mL). The maximum theoretical productions, obtained by the quadratic model, were 49.6 and 45.7 U/mL, and optimal temperatures of 28.3 and 30.2°C for U15-9 and U9-1, respectively. Thus, the maximum laccase production values and optimal temperatures are close to the theoretical values making evident that the adjustment of the quadratic model was effective to demonstrate the effect of temperature on the enzyme activity. Comparatively, U15-9 produced more laccase than U9-1 at 22, 25 and 28°C (P ≤ 0.05), but when the temperature increased from 28°C to 37°C, U9-1 laccase production reduced 30% while the U15-9 laccase production reduced 88%, indicating a great inhibition of the enzymatic activity of U15-9 at higher temperatures. The cultivation temperature affected the increasing mycelial biomass production quadratically for the strains (Figure 2). The increase in the mycelial biomass for U9-1 occurred when the temperature went up from 25°C to 28°C whereas for U15-9 it increased from 28 to 37°C. The mycelial biomass production of U9-1 was similar ranging from 22°C to 25°C and from 28°C to 37°C. However, for U15-9, the mycelial biomass production was similar ranging from 22 to 28°C, but there was an expressive increment in mycelial biomass from 28°C to 37°C. At 37°C, U15-9 produced 225% more mycelial biomass than at 28°C, and 108% more mycelial biomass than U9-1 at 37°C (Figure 2).
Figure 2: Effect of cultivation temperature on laccase and mycelial biomass production (average ± standard deviation) by Lentinus crinitus strains (U9-1 and U15-9). Uppercase letters indicate differences among laccase production and lowercase letters indicate differences among mycelial biomass growth according to Scott-Knott test (P ≤ 0.05).
Discussion
Our results showed that the maximum theoretical enzymatic activity for L. crinitus 15-9 was 47.8 U/mL and optimal pH of 5.6. Thurston (1994) reported that the initial pH of the cultivation medium from 4.5 to 6 favours laccase production by fungi. Prasad et al. (2005) evaluated the effect of the initial pH of the cultivation medium (From 4 to 8) on laccase production by Pleurotus ostreatus immobilized in polyurethane foam and defined cultivation medium. For these authors, the enzyme activity increased 167% when the cultivation medium pH changed from 4 to 5.5, but the pH values over 5.5 caused opposite effect in enzymatic production. Sivakumar et al. (2010) cultivated Ganoderma sp. in liquid medium containing starch as carbon source and initial pH between 4.5 and 6.5 and reported greater laccase production in pH 6. Strong (2011) cultivated Trametes pubescens utilizing full-strength distillery wastewater with pH adjusted from 3.5 to 6, and observed great variation of laccase production, but when the initial pH was 5 there was greater enzyme production. Therefore, based on our study and the reports in the literature, the initial pH of the cultivation medium is from 5 to 6 for greater laccase production by basidiomycetes. The medium pH can alter the cell morphology and fungus metabolism (Lee et al. 2013). Many of the fungus responses to pH changes are regulated by the Pal/Rim signaling pathway and the most studied ones include enzyme secretion and secondary metabolite synthesis (Aréchiga-Carvajal and Ruiz-Herrera, 2005; Wu et al. 2016). This regulatory genetic system guarantees that molecules exposed to the environment such as extracellular enzymes be produced only under pH conditions that allow their action effectively, indicating the adaptation capacity of these microorganisms (Peñalva et al., 2008). Therefore, based on the literature that indicates a greater laccase production at pH 5.0 to 6.0 and in our results with greater activity at pH 5.5 it is suggested that the enzymatic activity of laccase by basidiomycetes be also in this pH range.
Studies on the gene regulation of fungal responses to pH involve ascomycetes and few basidiomycetes, and the only report on the function of this system in higher basidiomycetes was for Ganoderma lucidum (Wu et al. 2016). In G. lucidum, a homolog of pH-responsive transcription factor PacC/Rim101 is involved in the mycelial growth, fruiting body development and ganoderic acid biosynthesis. No studies on the gene regulation response to pH in L. crinitus were found, but we can suppose that regulatory systems similar to the ones already described for other fungi may be involved in the regulation of complex physiological responses of this species.
The strains of L. crinitus grew under all the evaluated initial pH conditions. The initial pH of the cultivation medium affects the growth of fungi because it alters the cellular functions, morphological characteristics, uptake of nutrients and metabolites biosynthesis (Fang and Zhong 2002). However, the great diversity of cultivation conditions makes the comparison of results difficult. Maki et al. (2001) evaluated the effect of initial pH (between 5 and 7) of potato broth in Lentinula edodes mycelial biomass production.
Joshi et al. (2013) cultivated Schizophyllum commune in defined culture medium (2% glucose; 0.2% NaNO3; 0.05% yeast extract; 0.1% KH2PO4;0.05% MgSO4.7H2O and 0.05% KCl) with initial pH from 3 from 8 and observed maximum production of mycelial biomass at initial pH 6. Although the temperature is an important environmental factor, few studies approach the laccase production variation at different temperatures. Previous reports suggested that temperatures which favor the maximum laccase production ranged from 25°C to 30°C and temperatures higher than 30°C reduced enzyme production (Brijwani et al. 2010). Our study is in accordance with those reports since the greatest L. crinitus laccase production occurred between 25°C and 28°C for both strains.
Our study on the mycelial biomass production in function of the temperature shows that L. crinitus is a versatile and robust species, and that it tolerates temperature variations during the growth. This result is comparable to the growth of several basidiomycete species. Besides L. crinitus having produced great amounts of mycelial biomass at greater temperatures (37°C) than the ones expected for the ideal growth of macromycetes since the reports indicate that the ideal temperature for the growth of these fungus vary from 20°C to 30°C (Deacon 2006). Dulay et al. (2015) verified the maximum mycelial biomass production of Lentinus sajor-caju and Lentinus tigrinus in rice-broth at 32°C. Thus, the production capacity of mycelial biomass at temperatures higher than 30°C seems to be a characteristic of Lentinus genera. Our study is the first report on the ideal conditions of pH and temperature for laccase and mycelial biomass production by L. crinitus. These results are important to redirect the production strategies of L. crinitus and for the general knowledge on basic conditions of this fungus cultivation. We also verified that both L. crinitus strains presented distinct responses of enzyme and mycelial biomass production in face to the same environmental stimuli and that the laccase production is strain-dependent for L. crinitus.
The strain-dependent response for laccase production is described for other basidiomycetes. When two strains of three species of white-rot basidiomycetes were growth in mandarin peel cultivation medium, laccase production was species-dependent and strain-dependent (Elisashvili and Kachlishvili, 2009). For these authors, laccase activity was from 19.4 to 25.2 U/mL for Cerrena máxima, from 20.8 to 75.4 U/mL for Ganoderma lucidum, and from 7.3 to 16.3 U/mL for Trametes versicolor. Similarly, when four strains of Agaricus subrufescens were cultivated in the presence of different enzymatic inducers, laccase production varied from 2 to 29 U/mL (Valle et al., 2015). The variation of responses to environmental stimuli among the strains makes evident that intra-specific variations are common in basidiomycetes. The diversity of responses to L. crinitus strains suggests a genetic diversity among strains and species versatility to adapt to different environmental conditions, showing important characteristics to produce enzymes of biotechnological and industrial interest.
CONCLUSION
The initial pH from 5 to 7 in the cultivation medium does not affect the mycelial biomass production of Lentinus crinitus but affects the laccase production depending on the strains. The cultivation temperature affects mycelial biomass production and laccase production positively. The mycelial biomass production at 37°C of U15-9 is twice as high as U9-1; however, the laccase production at 37°C is drastically reduced mainly for U15-9 strain. The optimal values for laccase production of U9-1 are at pH 7 and 28°C, and for U15-9 are at pH 6 and 28°C.
Acknowledgments
The authors thank Universidade Paranaense, Graduate Program in Biotechnology Applied to Agriculture of Paranaense University, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support and fellowship.
About the Authors
Corresponding Author
J.S. Valle
Programa de Pós-Graduação em Biotecnologia Aplicada à Agricultura, Universidade Paranaense (UNIPAR), Umuarama-PR, Brazil
- Email:
- luziadoretto@prof.unipar.br
References
- Abraham WR, Abate D (1995). Chromanones from Lentinus crinitus (basidiomycetes). Z Naturforsch C. 50c: 748-750.
- Aréchiga-Carvajal ET, Ruiz-Herrera J (2005). The RIM101/pacC homologue from the Basidiomycete Ustilago maydis is functional in multiple pH-sensitive phenomena. Eukaryot. Cell 6: 999-1008. https://doi.org/10.1128/ec.4.6.999-1008.2005
- Brijwani K, Rigdon A, Vadlani PV (2010). Fungal laccases: production, function, and applications in food processing. Enz. Res. 2010. doi:10.4061/2010/149748. https://doi.org/10.4061/2010/149748
- Cambri G, Sousa MML, Fonseca DM, Marchini F, et al. (2016). Analysis of the biotechnological potential of a Lentinus crinitus isolate in the light of its secretome. J. Proteome Res. 15: 4557-4568. https://doi.org/10.1021/acs.jproteome.6b00636
- Cervantes-Chávez JA, Ortiz-Castellanos L, Tejeda-Sartorius M, Scott G, et al. (2010). Functional analysis of the pH responsive pathway Pal/Rim in the phytopathogenic basidiomycete Ustilago maydis. Fungal Genet. Biol. 47: 446-457. https://doi.org/10.1016/j.fgb.2010.02.004
- Chang ST, Miles PG (1997). Mushrooms: Cultivation, nutritional value, medicinal effect, and environmental impact. (2ndedn), CRC Press, Boca Raton.
- Deacon JW (2006). Fungal biology. (4thedn), Blackwell Publishing, Oxford.
- Dulay RMR, Flores KS, Tiniola RC, Marquez DHH, et al. (2015). Mycelial biomass production and antioxidant activity of Lentinus tigrinus and Lentinus sajor-caju in indigenous liquid culture. Mycosphere 6: 659-666. https://doi.org/10.5943/mycosphere/6/6/2
- Elisashvili V, Kachlishvili E (2009). Physiological regulation of laccase and manganese peroxidase production by white-rot Basidiomycetes. J. Biotechnol. 144: 37-42. https://doi.org/10.1016/j.jbiotec.2009.06.020
- Fang QH, Zhong JJ (2002). Effect of initial pH on production of ganoderic acid and polysaccharide by submerged fermentation of Ganoderma lucidum. Process Biochem. 37: 769–774.
- Gomes-Silva AC, Gibertoni TB (2009). Revisão do herbário URM: novas ocorrências de Aphyllophorales para a Amazônia brasileira. Rev. Bras. Bot. 32: 587-590.
- Groposo C, Loguercio-Leite C (2005). Contribution to the lignocellulolytic fungi (Basidiomycetes) of the Atlantic Rain Forest in Southern Brazil. Mycotaxon 92: 103-106.
- Joshi M, Patel H, Gupte S, Gupte A (2013). Nutrient improvement for simultaneous production of exopolysaccharide and mycelial biomass by submerged cultivation of Schizophyllum commune AGMJ-1 using statistical optimization. 3 Biotech. 3: 307-318. https://doi.org/10.1007/s13205-012-0103-3
- Kirsch L de S, Ebinuma V de C, Teixeira MF (2013). Mycelial biomass and biochemical properties of proteases produced by Lentinus citrinus DPUA 1535 (Higher Basidiomycetes) in submerged cultivation. Int. J. Med. Mushrooms 15: 505-515. https://doi.org/10.1615/intjmedmushr.v15.i5.80
- Lee JS, Jung WC, Park SJ, Lee KE, et al. (2013). Culture conditions and medium components for the production of mycelial biomass and exo-polysaccharides with Paecilomyces japonica in liquid culture. J. Biosci. Bioeng. 115: 433-437. https://doi.org/10.1016/j.jbiosc.2012.10.022
- Machado ARG, Teixeira MFS, Kirsch LS, Campelo MCL et al. (2016). Nutritional value and proteases of Lentinus citrinus produced by solid state fermentation of lignocellulosic waste from tropical region. Saudi J. Biological Sci. 23: 621-627. https://doi.org/10.1016/j.sjbs.2015.07.002
- Maki CS, Teixeira FF, Paiva E and Paccola-Meirelles LD (2001). Analyses of genetic variability in Lentinula edodes through mycelia responses to different abiotic conditions and rapid molecular markers. Braz. J. Microbiol. 32: 170-175. https://doi.org/10.1590/s1517-83822001000300002
- Meniqueti AB (2016). Condições de cultivo de basidiomicetos na bioacumulação de ferro. Master’s thesis, UNIPAR, Umuarama.
- Peñalva MA, Tilburn J, Bignell E, Arst Jr HN (2008). Ambient pH gene regulation in fungi: making connections. Trends Microbiol. 16: 291-300. https://doi.org/10.1016/j.tim.2008.03.006
- Pereira AM (2015). Seleção de basidiomicetos produtores de fitase por cultivo com resíduos agroindustriais. Master’s thesis, UNIPAR, Umuarama.
- Prasad KK, Mohan SV, Bhaskar YV, Ramanaiah SV, et al. (2005). Laccase production using Pleurotus ostreatus 1804 immobilized on PUF cubes in batch and packed bed reactors: Influence of culture conditions. J. Microbiol. 43: 301-307. https://doi.org/10.1016/j.bej.2005.01.019
- Silva GT, Gibertoni TB (2006). Aphyllophorales (Basidiomycota) em áreas urbanas da região metropolitana de Recife, PE, Brasil. Hoehnea 33: 344-543.
- Sivakumar R, Rajendran R, Balakumar C, Tamilvendan M, et al. (2010). Isolation, screening and optimization of production medium for thermostable laccase production from Ganoderma sp. Int. J. Eng. Sci. Technol. 2: 7133-7141.
- Strong PJ (2011). Improved laccase production by Trametes pubescens MB89 in distillery wastewaters. Enz. Res. https://doi.org/10.4061/2011/379176
- Thurston CF (1994). The structure and function of fungal laccases. Microbiology+ 140: 19-26.
- Umeo SH, Souza GPN, Rapachi PM, Garcia DM, et al. (2015). Screening of basidiomycetes in submerged cultivation based on antioxidant activity. Genet. Mol. Res. 14: 9907-9914.
- Valle JS, Vandenberghe LPS, Oliveira ACC, Tavares MF, et al. (2015). Effect of different compounds on the induction of laccase production by Agaricus blazei. Genet. Mol. Res. 8: 939-946. https://doi.org/10.4238/2015.december.1.40
- Valle JS, Vandenberghe LPS, Santana TT, Almeida PH, et al. (2014). Optimum conditions for inducing laccase production in Lentinus crinitus. Genet. Mol. Res. 13: 8544-8551. https://doi.org/10.4238/2014.october.20.31
- Wu F-H, Zhang G, Ren A, Dang Z-H, et al. (2016). The pH-responsive transcription factor PacC regulates mycelial growth, fruiting body development, and ganoderic acid biosynthesis in Ganoderma lucidum. Mycologia 108: 1104–1113.
Keywords:
Download:
Full PDF- Share This