Molecular Diagnosis of X-Fragile Syndrome: Perspectives for the Public Health System in the Central Region of Brazil
Received: October 14, 2017
Accepted: November 08, 2017
Published: December 15, 2017
Genet.Mol.Res. 16(4): gmr16039848
DOI: 10.4238/gmr16039848
Abstract
X-Fragile Syndrome (FXS) is the most common cause of inherited intellectual disability and the second of genetic origin, with an estimated prevalence of 1/4000 men and 1/6000 women. The etiology is associated with a trinucleotide expansion of CGG sequences and hypermethylation of the promoter region of the FMR1 (Fragile-X Mental Retardation-1) gene, located in the Xq27.3 region. Symptoms occur due to lack of Fragile X Mental Retardation Protein (FMRP), essential for dendrites growth and synaptic function. This syndrome is commonly underdiagnosed in children and adolescents due to the high phenotypic variability of patients and the need for a complex and high cost laboratory diagnosis. This research aims to evaluate individuals referred by the public health system of Goiás presenting intellectual deficiency suggestive of FXS and submitted to molecular diagnosis by PCR. Thirty-one patients, ranging in age from 4 to 41 years, were analyzed. It was possible to detect molecular alterations in the FMR1 gene in 6 patients with complete mutations, consistent with the observed phenotypes. The use of molecular techniques associated with capillary electrophoresis in an automatic genetic analyser demonstrated rapid and efficient investigation of CGG repeats in the FMR1 gene. Its inclusion in the public health service, in addition to universalizing access to genetic tests in Brazil, bridging the gap between effective diagnosis and the technologies available until now, has supported families in clarifying the etiology and assessing the risk of recurrence through genetic counselling
Introduction
The X-Fragile Syndrome (FXS) was first described in 1943 by Martin and Bell, after analyzing a family composed of eleven men who had severe cognitive deficits, children of normal mothers. After the structuring of the family heredogram, it was suggested that it was an X-linked inheritance disease, characterizing the form of transmission between members of the same family (Linhares et al., 2012). It is estimated that 3% of the world population has some kind of cognitive deficit, 40% of which is associated with X chromosome abnormalities. FXS is the main cause of mental deficiency inherited in the world, with an estimated frequency of 1: 4000 men and 1: 6000 women (France et al., 2011, Gigonzac et al., 2016). Despite its high prevalence, FXS remains poorly understood and underdiagnosed by health professionals, mainly pediatricians and family physicians (Goméz and Alonso, 2016).
Most cases of FXS originate from the expansion of CGG trinucleotides in the 5 'untranslated region of the FMR1 gene (Saldarriaga et al., 2014). Above 200 CGG repeats the gene tends to be methylated, preventing its transcription and synthesis of the FMRP protein (Bagni et al., 2012). This protein is responsible for several biological functions in the brain, controlling the gene expression in the signaling of several receptors (Felix and Pina-Neto, 1998). According to França et al. (2012), any alteration of the FMR1 gene that results in the absence or deficit of the FMRP protein, would lead to the reduction of synaptic plasticity, fundamental to the learning and memory processes.
FXS presents a broad spectrum of clinical manifestations, such as intellectual deficit, autism spectrum disorder, anxiety and withdrawal, language deficits, hyperactivity, aggressiveness, and self-aggressive behaviors. As for the morphological aspects, the presence of prominent articular hyperextensibility, prominent forehead and brow, high palate, large ears and macroorchidism at puberty are highlighted (Costa et al., 2006; River et al., 2010; Kwok et al. 2016).
Until 1991, the gene responsible for FXS was unknown and the diagnosis was based only on cytogenetic techniques (Pastori et al., 2014). However, these tests are still ineffective in identifying less than 5% of the patients, in addition to not identifying the extent of CGG repeats (Costa et al., 2006; França et al., 2011). With the advancement of molecular techniques, it was possible to diagnose using the Southern Blot technique, through which it is possible to specify the approximate degree of expansion. However, it also presents limitations, since it is a very laborious method, which requires a long time to be executed, requires a large amount of biological material, and yet does not have good diagnostic accuracy (Bagni et al., 2012). More recently, molecular analysis based on the Polymerase Chain Reaction (PCR) has been more successful in diagnosis, being a faster, more accurate method and using a minimum amount of biological material. It is estimated that around 10 hours it is possible to execute the whole process, from DNA extraction, amplification, fragment separation and analysis (Esperón Álvarez et al., 2015).
Until 2014, there was no routine for the molecular diagnosis for FXS in the state of Goiás under the Unified Health System (SUS), which implied a lack of regional epidemiological data on the syndrome, besides a serious problem for all patients who depend of the public health service. By mid-2015, the state pioneered the implementation of a new methodology for a rapid, accurate, and effective diagnosis of FXS (Gigonzac et al., 2016). Despite this great step for laboratory diagnosis, there is still a great difficulty in the identification and referral of patients due to the wide phenotypic variability of the disease, which shares characteristics with several other disorders. Also important is the early diagnosis of FXS in order to allow more effective and specific therapeutic interventions, besides allowing the targeting to the genetic counseling service, because it is a inheritable disease that can strongly influence the reproductive decision of the family involved ( (Edwards et al., 2004). Thus, the objective of this study was to evaluate individuals referred by the public health system of Goiás presenting intellectual deficiency suggestive of FXS and submitted to molecular diagnosis to identify specific mutations in the FMR1 gene.
Materials and Methods
This is a retrospective cross-sectional study carried out by the Laboratory of Human Cytogenetics and Molecular Genetics (LaGene-Lacen) of the State Department of Health of Goiás (SES-GO). The work was submitted and approved by the Ethics and Research Committee (CEP) of PUC-GO, under the number 1.990.981. The medical records of patients with intellectual disability and clinical indication of FXS were analyzed and sent to the laboratory from August 2015 to July 2016.
Samples were obtained by venipuncture and DNA extracted using the commercial reagent kit QIAamp®DNA Blood Mini Kit (Qiagen®), following the manufacturer's recommendations. The obtained DNA was quantified in NanoVue™ Plus (GE Healthcare Life Sciences®) spectrophotometer and diluted to a concentration of 20 ng/μL.
Amplification of the promoter region of the FMR1 gene was performed using the AmplideX® FMR1 PCR reagent set (Asuragen, Austin, Texas). In order to guarantee the amplification of long CGG repeats, which are generally refractory to amplification, a Triplet-Primed PCR (TP-PCR) was performed, in addition to the pair of forward and reverse primers, a third primer that is annealed in the CGG tape extension.
The amplification products were subjected to capillary electrophoresis in an ABI3500 automatic genetic analyzer (Applied Biosystems, USA), according to the protocol established by Gigonzac et al. (2016) by adjusting the polymer density of the capillary and all conditions for separating the fragments. The obtained electropherograms were analyzed using GeneMapper® software, version ID-X 1.4 (Applied Biosystems, Vermon Hills, Illinois, USA), allowing the determination of the size of the fragments of each patient.
Genotyping, given by the determination of the number of CGG repeats (CGGn), was performed based on the observed size of the amplicons (To) with the reduction of the common size for all the fragments (C0), and was then divided by the pattern derivation factor linear migration of the ROX1000 relative to the CGG cracking moiety (M0): Genotype (CGGn) = T0 - C0/M0 ≈ T0 - 230/2,995. In this formula, the size of the fragments generated on the electropherograms and characterized in base pairs (bp) are converted into alleles and named according to the amount of CGG repeats present.
Results
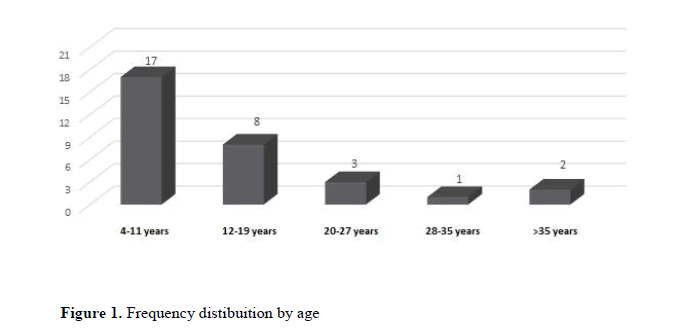
In the initial phase of the implementation of the molecular analysis, 31 patients with suspected X-Fragile Syndrome were referred. Of these patients, 11 were female (35.5%) and 20 were male (64.5%). The patients' age ranged from 4 to 41 years, with the mean age being 13 years. The frequency distribution by age group revealed that most of the referrals were between 4 and 11 years old (Figure 1).
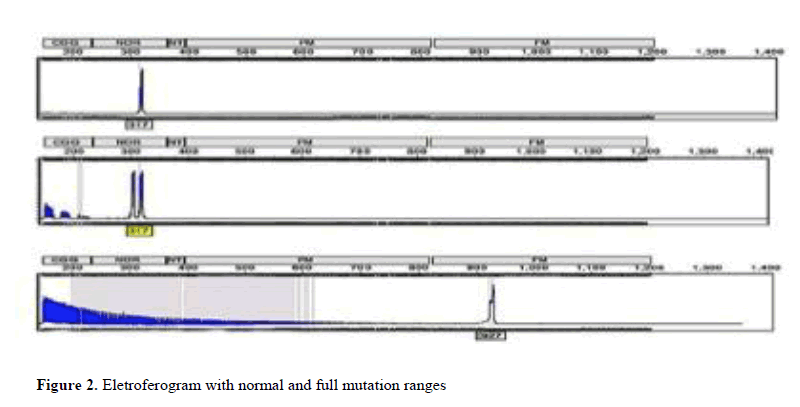
As for molecular analysis, it was possible to identify individuals presenting variations in the number of CGG repeats within the normal range (repetitions between 6 to 44), with pre-mutation (repetitions between 55 and 200) and complete mutation (over 200 replicates), as observed in Figure 2.
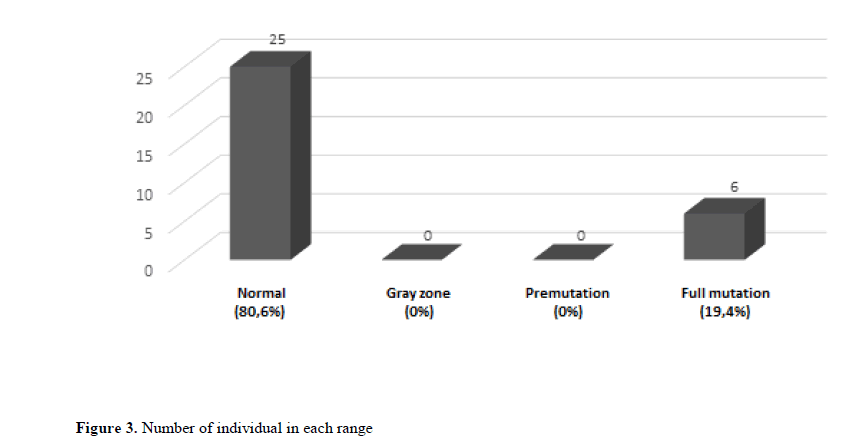
Among the 31 patients analyzed, 25 patients had less than 45 repetitions, therefore normal individuals for FXS, which corresponds to 80.6% of the total sample. No individuals were identified in the pre-mutation zone or the gray zone. Of the remainder, 5 males (83.3% of the total group of individuals with a mutation) and one woman (16.7% of the total group of individuals with a mutation) had complete mutations (Figure 3).
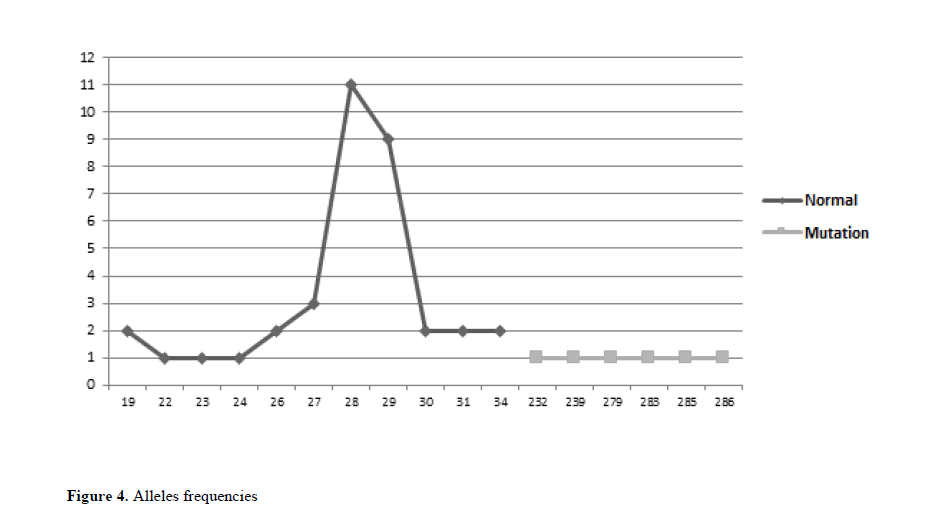
As for the number of replicates of the analyzed alleles, a variation of 19 to 286 CGG replicates was observed. In this study, the most frequent replicates in the normal individuals group were 28 CGG replicates, comprising 11 patients, and 29 replicates, comprising 9 patients. Among individuals with a complete mutation, there are no a prevalent number of replicates, and in each of the six patients with a mutated X chromosome there are a number of specific CGG repeats in the range of 280 to 290 replicates (Figure 4).
Of the 31 patients with intellectual disability suggestive of FXS referred for diagnosis, 6 had their diagnosis confirmed which corresponds to 19.4% of the total referrals and reinforces FXS as a major cause of intellectual disability (Figure 5).
Discussion
Molecular separation methods proposed by capillary electrophoresis for the identification of mutations in the FMR1 promoter region have been shown to be more efficient than other methodologies, such as by Southern blot, by accurately categorizing mutation ranges and mainly by detecting mutation amplicons which are generally present in very low amounts to be identified by conventional electrophoresis. (Gigonzac et al., 2016)
The fact that the sample presents more male patients than the female one is justified because it is a disease with dominant inheritance linked to the X chromosome, affecting more men than women. The phenomenon of random inactivation of this chromosome, in the female sex, also inactivates part of the mutated X chromosomes, with consequent reduction of clinical manifestations of the disease (Willemsen et al., 2001). Although the symptomatology of FXS in women is less common, the process of unfavorable random inactivation may justify the phenotype (Marshall et al., 2013).
The occurrence of males with a complete mutation more frequently than in females corroborates data from Noto et al. (2016) which indicates the estimated prevalence of FXS being higher in males than in females, a fact observed in received for laboratory diagnosis.
The most frequent alleles in the studied sample were within the range of normality, with 28, 29 and 30 repetitions, finding compatible with several studies that raised the number of repetitions more common1 (Crawford et al., 2001; Terracciano et al. 2004, Wheeler et al., 2017). On the other hand, the determination of the frequency of mutated alleles is not usual since they tend to modify and increase from generation to generation by slippage mechanisms (Madallena et al., 2001; Fernandez-Carvajal et al., 2009)
When analyzing other similar studies to diagnose the syndrome, it was observed that the study by Boy et al. (2001), analyzed in the Human Genetic Service of the State University of Rio de Janeiro, a group of 104 individuals with mental retardation idiopathic, a complete mutation was diagnosed in 17 of them, obtaining a frequency of 16.3% of the total, which corroborates with the results found in our study. Another comparative study was carried out by Silveira et al. (2015) in which a sample of 106 patients referred for molecular investigation by intellectual deficit was analyzed, of which 37 were submitted to the diagnosis of FXS. Another 30 patients did not present any other suspicion and therefore they were analyzed for the syndrome and 7 had a specific suspicion of FXS. Five patients were confirmed as having the mutation (seven of them suspected of FXS, four of them had the mutation). When analyzing these data, 13.5% of the patients submitted to the specific diagnosis for FXS had the confirmation of the mutation, something compatible with the findings of our referrals for specific research for FXS.
Compared with the study by Oliveira et al. (2004), who analyzed 25 patients from the Municipal School of Autism "Maria Lúcia de Oliveira" in São José do Rio Preto, only 1 was diagnosed with a complete mutation using the PCR technique at a frequency of 4%. The patients were submitted to molecular diagnosis, with a methodology that can now be considered superior to the other previously used tests, such as cytogenetics and Southern Blot, since it allows detecting the expansion of the FMR1 gene and identifying the type of mutation present (Steiner et al., 2005; Amâncio et al., 2015).
In Brazil, the Unified Health System has sought technological solutions that, considering the socio-economic complexity, are necessary to obtain efficiency and quality in care. Thus, the molecular method implemented allowed access to accurate and effective diagnosis that was not available by the public health system. In addition, the identification of specific mutations in the analyzed individuals contributed to the clarification and orientation of the families through the genetic counseling service. It was then possible to provide explanations about the prognosis and risk of recurrence of the alteration, being these fundamental aspects for understanding the course of the disease, choosing appropriate therapeutic interventions and reproductive decisions.
Conclusion
The implementation of methodologies for molecular diagnosis using capillary electrophoresis, with technical quality, smaller amount of biological material, associated to the use of the latest generation genetic analyzers, has been shown to be effective in the diagnosis of FXS. This progress allows rapid and reliable results which will influence the possibility of more specific therapies and genetic counseling of patients, either with the complete mutation or with the risk of pre-mutation, with possible recurrence in subsequent generations. The impact of the implementation of the molecular diagnosis of FXS in the public health system should generate new perspectives on the knowledge of the syndrome for the State and, especially, on the approach of genetic counseling, which is based on a detailed investigation that goes from the elaboration of a heredogram and collection of the family history, until the subsequent identification of the possible individuals who inherited the mutation of the FMR1 gene. This behavior should be initiated early, since it allows the genetic counselor to communicate to the family about the reality of the disease, to provide knowledge about the transmission of FXS and its consequences, besides being able to present advanced and specific therapies, which helps in the planning family relationship in a conscious way and in the possibility of preventing the recurrence of the syndrome in the affected families.
Acknowledgments
This work was supported by LaGene-Secretary of Goiás State for Public Health and Replicon Research Center, Pontifical Catholic University of Goiás (PUC-GO).
About the Authors
Corresponding Author
T.C. Vieira
Faculty of Medicine, Pontifical Catholic University of Goiás - PUC-GO, Goiania, Goiás, Brazil
- Email:
- thaiscidalia@gmail.com
References
- Abrams L, Cronister A, Brown WT, Tassone F, et al. (2012). Newborn, carrier, and early childhood screening recommendations for fragile X. Pediatrics. 2012 Dec 1;130 (6):1126-35. https://doi.org/10.1542/peds.2012-0693
- Amancio AP, Melo CA, Vieira A, Minasi L.B, et al. (2015). Molecular analysis of patients suspected of Fragile X Syndrome. Genetics and Molecular Research, v. 14:14660-14669. https://doi.org/10.4238/2015.november.18.30
- Bagni C, Tassone F, Neri G, Hagerman R. (2012). Fragile X syndrome: causes, diagnosis, mechanisms, and therapeutics. The Journal of clinical investigation. Dec 3;122(12):4314-22. https://doi.org/10.1172/jci63141
- Costa SS, Fonseca AM, Bagnoli VR, Vianna-Morgante AM. (2006). The FMR1 premutation as a cause of premature ovarian failure in Brazilian women. Genetics and Molecular Biology. 29(3):423-8. https://doi.org/10.1590/s1415-47572006000300002
- Crawford DC, Acuña JM, Sherman SL. (2001). FMR1 and the fragile X syndrome: human genome epidemiology review. Genetics in Medicine. Sep 1:3(5):359-71. https://doi.org/10.1097/00125817-200109000-00006
- Debrey SM, Leehey MA, Klepitskaya O, Filley CM, et al. (2016). Clinical phenotype of adult fragile X gray zone allele carriers: a case series. The Cerebellum. Oct 1;15(5):623-31. https://doi.org/10.1007/s12311-016-0809-6
- Esperón-Álvarez AA, Merencio SL, López RI, Reyes NL, et al. (2015). Diagnóstico molecular del síndrome de X frágil mediante PCR específica de metilación. Revista Cubana de Genética Comunitaria. Aug 28;9(2):17-23.
- Felix T, Pina-Neto JM. (1998). Fragile X syndrome: clinical and cytogenetic studies. Arquivos de neuro-psiquiatria. Mar;56(1):09-17. https://doi.org/10.1590/s0004-282x1998000100002
- Fernandez-Carvajal I, Walichiewicz P, Xiaosen X, Pan R, et al. (2009). Screening for expanded alleles of the FMR1 gene in blood spots from newborn males in a Spanish population. The Journal of Molecular Diagnostics. Jul 31;11(4):324-9. https://doi.org/10.2353/jmoldx.2009.080173
- Finucane B, Abrams L, Cronister A, Archibald AD, et al. (2012). Genetic counseling and testing for FMR1 gene mutations: practice guidelines of the national society of genetic counselors. Journal of genetic counseling. Dec 1;21(6):752-60. https://doi.org/10.1007/s10897-012-9524-8
- França DC, Maria DM, Correa IP, Aburad AT, et al. (2011) Síndrome do X Frágil: relato de caso. Revista Faipe. Feb 22;1(1):1. https://doi.org/10.5123/s0101-59072006000400009
- Gigonzac MA, Teodoro LS, Minasi LB, Vieira TC, et al. (2016). Standardization of capillary electrophoresis for diagnosis of fragile X syndrome in the Brazilian public health system. Electrophoresis. Nov 1;37(23-24):3076-8. https://doi.org/10.1002/elps.201600333
- Gómez BM, Alonso IG. (2016). Síndrome x frágil: deteccióne intervention en el fenotipo conductual. International Journal of Developmental and Educational Psychology. 2(1):145-154. https://doi.org/10.17060/ijodaep.2014.n1.v2.427
- Kwok YK, Wong KM, Lo FM, Kong GW, et al. (2016). Validation of a robust PCR-based assay for quantifying fragile X CGG repeats. Clinica Chimica Acta. May 1;456:137-43. https://doi.org/10.1016/j.cca.2016.02.027
- Linhares ND, Svartman M,Valadares ER. (2012). Diagnóstico citogenético de pacientes com retardo mental idiopático. J Bras Patol Med Lab. Feb;48(1):33-9. https://doi.org/10.1590/s1676-24442012000100007
- Maddalena A, Richards CS, McGinniss MJ, Brothman A, et al. (2001). Technical standards and guidelines for fragile X. Genetics in Medicine. May 1:3(3):200-5.
- Marshall LS, Simon J, Wood T, Peng M, et al. (2013). Deletion Xq27. 3q28 that includes IDS and FMR1 in female patient with global developmental delays and nonrandom X inactivation. BMC Medical Genetics. January 1(1): 92-97. https://doi.org/10.1186/1471-2350-14-49
- Noto V, Harrity C, Walsh D, Marron K. (2016). The impact of FMR1 gene mutations on human reproduction and development: a systematic review. Journal of assisted reproduction and genetics. 33(9): 1135-1147. https://doi.org/10.1007/s10815-016-0765-6
- Oliveira AB, Giunco CT, Carvalho AB, Salles F, et al. (2004). Investigação molecular por PCR da síndrome do cromossomo X frágil em homens com transtornos invasivos do desenvolvimento. Arq Ciênc. Saúde. 11(1):25-8.
- Pastori C, Peschansky VJ, Barbouth D, Mehta A, et al. (2014). Comprehensive analysis of the transcriptional landscape of the human FMR1 gene reveals two new long noncoding RNAs differentially expressed in Fragile X syndrome and Fragile X-associated tremor/ataxia syndrome. Human genetics. 133(1): 59-67. https://doi.org/10.1007/s00439-013-1356-6
- Rivera SM, Stebbins GT, Grigsby J. (2010). Radiological findings in FXTAS in the Fragile X-Associated Tremor Ataxia Syndrome. Springer New York. 55-66. https://doi.org/10.1007/978-1-4419-5805-1_4
- Saldarriaga W, Tassone F, González-Teshima LY, Forero-Forero et al. (2014). Fragile X syndrome. Colombia Médica. 45(4): 190-198.
- Silveira CS. (2015). Caracterização clínica e demográfica de pacientes em investigação diagnóstica para Síndrome do X-Frágil.
- Steiner CE, Guerreiro MM, Marques-de-Faria AP, Lopes-Cendes I. (2005). Laboratorial diagnosis of fragile-X syndrome: experience in a sample of individuals with pervasive developmental disorders. Arquivos de neuro-psiquiatria. Sep;63(3A):564-70. https://doi.org/10.1590/s0004-282x2005000400002
- Terracciano A, Pomponi MG, Marino GM, Chiurazzi P, et al. (2004). Expansion to full mutation of a FMR1 intermediate allele over two generations. European journal of human genetics. Apr 1;12(4):333-6. https://doi.org/10.1038/sj.ejhg.5201154
- Wheeler A, Raspa M, Hagerman R, Mailick AD et al. (2017). Implications of the FMR1 premutation for children, adolescents, adults, and their families. Pediatrics, 139 (Supplement 3), S172-S182. https://doi.org/10.1542/peds.2016-1159d
- Willemsen R, Levenga J, Oostra BA. (2011). CGG repeat in the FMR1 gene: size matters. Clinical genetics. Sep 1;80(3):214-25. https://doi.org/10.1111/j.1399-0004.2011.01723.x
Keywords:
Download:
Full PDF- Share This