Nanotechnology to treat sepsis
Received: June 20, 2024
Accepted: June 24, 2024
Published: June 28, 2024
Genet.Mol.Res. 23(2):
Keywords
Sepsis; Nanotechnology; Therapeutic efficacy; Mortality; Polymer sciences
Introduction
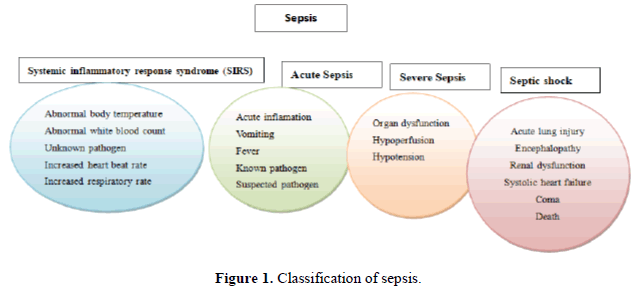
Sepsis is a life-threatening organ dysfunction disease triggered by a dysregulated host immune response to infection and has been known for over 2700 years [1]. In 2017, the World Health Organization (WHO) regarded sepsis as a critical unmet medical need [2]. To understand sepsis in practical and conceptual frameworks, the American College has defined the word SIRS (system inflammatory response for an infection) in various forms, as shown in Figure 1 in a consensus conference organized by Society of Critical Care Medicine (SCCM) at Chicago, USA. The definition of sepsis has provided criteria to design the inclusion criteria for conducting different clinical experiments or trials to understand better the aspects of the pathophysiological association with sepsis and its related pathological disorders (Figure 1).
A recent study has reported that sepsis incidences were severely underestimated and are at least double the previously predicted [3]. The annual burden of sepsis is expected to be nearly 48 million sepsis cases, with 11 million mortalities across the globe, representing approximately 19% of all global deaths. Although huge progress in early diagnosis and treatment, sepsis still has a mortality rate of nearly 30%, and effective drug treatments are unavailable at clinics [4]. Antibiotics are used as major cornerstones to treat sepsis; administering proper antibiotics, defined as those that exhibit in vitro activity against pathogens that cause infection, in a fast mode to gain sufficient drug concentration at the infection site can enhance the survival of patients with sepsis [5-7]. Nonetheless, prescribing correct antibiotics to sepsis patients is a major challenge for physicians because of infection risk due to multidrug-resistant bacteria. In addition, most standard antibiotics regimens are failed to provide suitable antibiotics concentration at the infection site [8]. The major factor which reduces antibiotics efficacy is the emergence of antibiotic resistance [9]. Therefore, identification of more effective treatment strategies is urgently needed to treat sepsis and provide a new hope to patients suffering from sepsis.
Literature Review
Molecular mechanism of sepsis pathophysiology
Sepsis is a lethal clinical syndrome with heterogeneous malignancy courses. Sepsis can be characterized by an altered response against infection induced by Pathogen-Associated Molecular Patterns (PAMPs) from insidious microorganisms by the innate immune system. PAMPs are extremely conserved fungal or bacterial origin. They are distinguished by four types of receptors: C-type lectin receptors, toll-like receptors, nucleotide-binding oligomerization domain-like receptors, and retinoic acid-inducible gene 1-like receptors [10]. The resulting inflammatory response led to the activation of complex intra-and extracellular cascades that mediate cell lysis and spillover of intracellular molecules towards extracellular space. Damage-Associated Molecular Patterns (DAMPs) can also be excreted following extensive tissue trauma, and behave like PAMPs on the host immune system, with induction of inflammation threat [11,12]. Along with, the body triggers a Compensatory Anti-Inflammatory Reaction (CARS) which results in an enhanced glucocorticoid release, a key inducer of anti-inflammatory cytokine such as IL-10 [13]. The net upshot of inflammatory pathway contributes to enhanced capillary permeability and vasodilation, thereby leading to hypotension. In sepsis, overexpression of tissue factors results in the downregulation of the anti-thrombin and a subsequent amplification in plasmathrombin. In addition, overexpression of plasminogen activator inhibitor type 1 and reduced production of protein C suppresses fibrinolysis. Collectively, these events mediate a hypercoagulable state. Hypotension and enhanced coagulation in sepsis contribute to multi-organ dysfunction, the most critical and life-threatening sepsis outcome [14]. Various inflammatory mechanisms are involved during sepsis, and several mechanisms have been studied for clinical use.
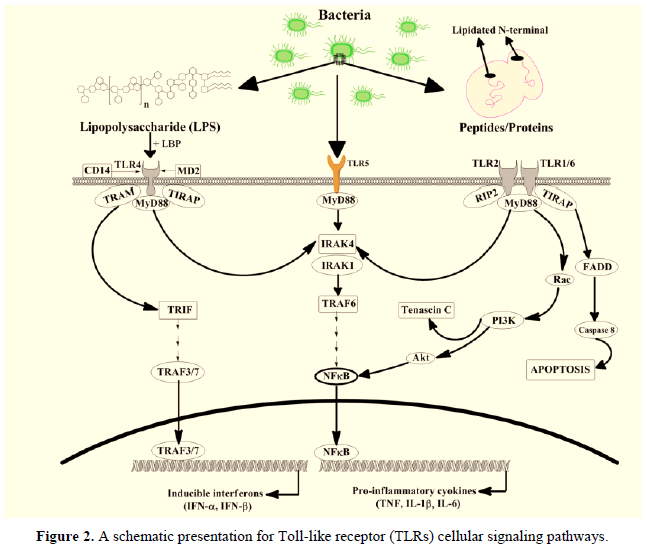
The Lipopolysaccharide (LPS) has been found as a major organic chemical component in the bacterial cell membrane of gram-negative bacteria. German scientist conducted research and coined the term ‘‘endotoxin’’ which are usually produced by micro-organisms (Figure 2). It has been found that the bacterial cell contains up to 2 million molecules of LPS, which covers up to nearly 75 % of the cell membrane surface. The LPS is a complex organic molecule comprising an O-specific chain (O-antigen), a core oligosaccharide, and a covalently bounded Lipid A moiety. The LPS has been found in the well-known Pathogen-Associated Bio-molecular Pattern (PAMP), which is well known to elicit its actions through a type of Pattern Recognizing Receptor (PRR) following the Toll-like receptor-4 (TLR4), expressed on the bacterial cells for maintaining the cell internal immune system. Activating the TLR4 through LPS is not a simple mechanism involving many important molecules which carry the LPS molecule to the TLR4. During the acute sepsis phase, a protein is produced in the liver cells where the LPS binding proteins mediate the decisive LPS recognition stage. The LPS, after binding, produced the LPS-LBP complex. The LPS-LBP complex is then recognized using the cluster differentiation 14 receptors, which binds it to create the complex tertiary compound. The TLR4 linker molecular dimerization occurs with the Myeloid Differentiation factor 2 (MD2) while bringing the intracellular Toll/interleukin-1 receptor or TIR linking molecular domains, which allows the binding of LPS with other cellular adaptor protein molecules.
The nuclear translocation of NF-jB and its activation usually ends up in the TLR4 binding and signaling as an adaptor protein molecule [15,16]. It also leads towards up-regulating pro-inflammatory cytokines, including IL-6, IL-1b, TNF-a, and IL-8 (Figure 2). It also involves other biomolecules and molecules such as E-Selectin, COX-2, iNOS, and MCP-1. It has been reported that monoclonal antibodies and LPS showed antagonistic effects against each other, leading TLR4 to block harmful activities [17,18]. At the same time, LPS from the cell membrane of bacterial cells became a poisonous compound to induce acute or severe types of sepsis and septic shock. It has also been discovered in another study on mice with a mutation in the Tlr4 gene (C57BL/10ScCr and C3H/HeJ) showed resistance to LPS action. At the same time, a very high susceptibility was found in gram-negative bacterial infection; it was suggested that there might be an involvance of some other biochemical in the bacterial membrane that elicit the inflammation disease [19-21].
The TLRs usually recognize bacterial cells and/or their cell products. The activation usually depends upon the MyD88. However, the activation of TLR2 and TLR4 started similarly during the activation for NFjB, along with highly notable variations between both pathways. The interferon (a/b) genes are predominantly upregulated through the activation of TLR4, the TLP2 is activated by the Casapase 8-dependent cellular apoptotic machinery. The Tenascin C, a glycoprotein, is upregulated in the response due to the activation of TLR2 while studying the in vitro models. The Lipopolysaccharide-Binding Protein (LBP), MD2 lymphocyte antigen 96, TIRAP Toll-Interleukin 1 Receptor (TIR) domain-containing adaptor protein, TLR toll-like receptor, Interleukin-1 Receptor-Associated Kinase (IRAK), CD-14, the TRAM toll-like receptor-4-adaptor protein, myeloid differentiation factor 88 (MyD88), nuclear factor jB (NF-jB), PI3K phosphoinositide 3-kinase, receptor-interacting protein 2 (RIP2), TIR-domain-containing adapter-inducing interferon-b (TRIF), IFN interferon, the Tumor necrosis factor Receptor-Associated Factor (TRAF) and the FADD was associated death domain protein.
Nanotechnology
Delays in sepsis treatment can impact patient’s clinical outcomes. More specific and fast tests are immediately required to avoid inappropriate, unnecessary, and ineffective antibiotics. Considering all pitfalls linked with sepsis, there is a critical need to establish fast, sensitive and pathogen-specific detection tools and novel antimicrobial approaches. Previously, various effective targets were suggested as potential sepsis detection and therapy tools. However, they were proved unfeasible for clinical implementation due to hurdles in modeling highly variable septic responses in preclinical systems [22]. Sepsis involves various pathological pathways; thus, there are not enough representative animal models that reflect sepsis's heterogeneity and sufficiently simulate its complexity. To date, limited preclinical data shows increased specificities and sensitivities compared with clinically employed tools, which poses a major challenge during clinical trials [23]. Advancement in nanotechnology and its application in medicine have revolutionized the traditional pharmaceutical industry and medical field [24]. This emerging field has already provided innovative solutions for improving diagnostic and therapeutic management of various pathologies [25,26]. Strikingly, although nanotechnology has emerged in the last few decades, above 200 nano-medicine constructs are being tested for clinical use [27]. Various nano-formulations have been developed and identified for possible treatment of sepsis.
Nanotechnology-based combination therapy
The complexity of sepsis pathophysiology with different signs and symptoms, such as microbial infection in infected areas leads to organ dysfunction. Thus, it is critical to eradicate this severe issue for the more effective treatment of sepsis. There is no debate that the delivery of a single drug is insufficient to treat sepsis, and a high dose of a single drug causes severe toxicity. In recent years, many scientists have investigated dual targeting strategies. A recent study has developed a novel combinatorial approach by co-administrating rutin and moxifloxacin loaded in polycaprolactone nanoparticles (PCL). In this novel approach, rutin has been used to down regulate cytokines production and reduce inflammation, while moxifloxacin has been used to kill bacteria. The lyophilized formation has been investigated in vitro in the J774 cell line. Research findings obtained from this study have validated the phagocytic action of the newly developed PCL nanoparticles platform and suggested that rutin and moxifloxacin are safe to use and may be used for combination therapy [28]. In another recent study, scientists have formulated an Antimicrobial Peptide (AMP) with cathepsin B in lysosomes incorporated in vitamin C nanocarriers for the adoptive translocation in the macrophages. In this study, cathepsin B has been used to transport AMPs into lysosomes, and AMP has been used to destroy bacteria. This novel combinatorial approach was developed to overcome multidrug resistance (MDR) bacteria tempted sepsis. Research findings obtained from in vivo experiments demonstrated that AMP conjugated with cathepsin B in lysosomes eliminates MDR bacteria, thereby promoting the recovery of septic mice [29].
Discussion
Identification of novel biomarkers in sepsis
Many promising diagnostics tools are being established to assist in managing septic patients. Developing novel diagnostics tools can allow early sepsis diagnosis, facilitating the characterization of the specific infecting organisms and molecular pathways that become dysfunctional in sepsis. Despite substantial development in our understanding of sepsis pathophysiology, no single sepsis biomarker has yet to address all the diagnostic needs. Previous studies have reported many host biomarkers [30]. However, none of them demonstrated a sufficient level of sensitivity and specificity [31]. Several new approaches have been used in distinguishing sepsis from sterile inflammation by using metabolomics, transcriptomics, or microfluidics [32-34]. In addition, combined analysis of leukocyte-based biomarkers has also been explored. Finally, transcriptomics and proteomics have also been reported to permit discrimination between viral and bacterial infections [35,36]. Identifying novel specific biomarkers is urgently needed to improve sepsis patients’ evaluation and treatment.
Blood cleansing devices
Magnetic separation is a tried-and-true method for isolating particular cells, chemicals, or contaminants from complicated mixtures such as whole blood or biological samples. In vitro and in vivo animal models have both used it to lower pathogen levels in the blood. Methods for treating blood using magnetic particles have several advantages over conventional methods, including a greater diffusion collision rate constant and bigger active surface areas for pathogen entrapment [37-39]. Additionally, unlike other techniques that call for repeated blood passages, which may gradually reduce their efficacy, these approaches permit continuous replenishment of capturing reagents in the bloodstream. The effectiveness of magnetic blood treatment in eliminating germs from septic blood depends on several factors. First, catching various infections without prior identification depends critically on choosing opsonin molecules [40,41]. Creating a universal opsonin molecule that targets various infections that express different protein or carbohydrate chains has been difficult. Researchers have explored using magnetic particles or metal chelators coupled to antibody molecules to remove germs or chemicals from the blood [42-44].
The size and number of magnetic particles are also significant factors. The blood cleaning system's effectiveness can be maximized by using ideal values. A theoretical model integrating collision and magnetophoretic principles has predicted the appropriate size and concentration of magnetic nanoparticles for efficient pathogen depletion in various blood components. A crucial element of magnetic separation is also played by the magnetic force acting on the complexes of magnetic particles linked to pathogens [44,45]. The gradients in magnetic flux density along the direction of magnetic tugging immediately affect the force. The magnetic forces can be turned on and off easily with electromagnetic devices, but the flux density gradients they can produce are constrained by electromagnetic heating. Alternately, permanent magnets with alternating polarisation can produce significantly increased flux density gradients, and magnetic nanoparticle manipulation using the Halbach magnetic array has been successfully demonstrated [46,47].
For manipulating super paramagnetic nanoparticles with weak magnetic moments, ferromagnetic microstructures have recently been used to increase the magnetic flux density gradients. Furthermore, even in pure whole blood, the magnetic separation efficiency has been markedly enhanced by novel microfluidic channel designs, including secondary spiral flows and magnetophoresis [45,46,48]. The magnetic separation principle could probably be applied to biomedical equipment to aid septic patients soon, given the success and persistent efforts in magnetically eliminating pathogens from whole blood.
Commercial products available in the market
Extracorporeal biomedical devices approved by regulatory authorities worldwide for septic patient treatment predominantly rely on surface-adsorption principles, as indicated in Table 1. The therapeutic efficacy of ToraymyxinTM filters has shown regional variations and inconsistent clarity. This has led to differences in pricing, with general health insurance providers in the Republic of Korea currently not subsidizing their use, while the Japanese national health insurance has been able to do so since 1994. Despite insufficient encouraging outcomes from Randomized Controlled Trials (RCTs) evaluating the adjunctive use of extracorporeal treatments, sporadic clinical studies have reported positive efficacy in treating septic patients, supporting the continued utilization of these devices in intensive care units [47,48]. Conducting RCTs for extracorporeal devices in sepsis treatment poses challenges, primarily due to the limited availability of eligible patients for participation. However, as the therapeutic efficacy of these devices demonstrates exceptional and unquestionable outcomes, this limitation may be resolved. Additionally, alongside the products listed in Table 1, numerous startup companies are actively pursuing the translation of their innovative approaches to clinical practice [49,50].
| Product name | Principle | Company | Country | Targets of removal |
|---|---|---|---|---|
| Seraph® | ExThera Medical | USA | Bacteria (MRSA, E.coli, ESBL-K. pneumoniae, VRE, etc.) | |
| Toraymyxin™ | Toray Industries | Japan | Gram-negative bacteria, endotoxin | |
| Under development | BOA Biomedical Inc.* | USA | A broad range of pathogens | |
| Alteco® LPS Adsorber | Alteco Medical AB | Sweden | Endotoxin | |
| oXiris® | Adsorption | Baxter Int. Inc. | USA | Endotoxins, cytokines, uremic toxin |
| Under development | Magnetic particle separation | hemotune* | Switzerland | Endotoxin, cytokines, drugs, heavy metals |
| Cytosorb® | CytoSorbents | USA | Cytokines |
Table 1. Regulatory approved extracorporeal biomedical devices for sepsis treatment and their therapeutic efficacy variations by region.
Technical and experimental challenges
The primary objective of extracorporeal adjuvant therapy is to reduce the levels of Pathogen-Associated Molecular Patterns (PAMPs) in the bloodstream of septic patients. Despite the availability of the Endotoxin Activity Assay (EAATM) since 2004, there is currently no internationally approved instrument for quantifying PAMP levels in whole human blood [51,52]. Recent research investigating pathogen sequencing in septic patient blood has revealed a considerable percentage of inaccurate results, particularly when low pathogen concentrations. In addition to depleting PAMPs, extracorporeal therapy's effectiveness is influenced by antibiotic efficacy, highlighting the importance of developing rapid diagnostic tools for antibiotic susceptibility testing. Clinicians must adopt modern diagnostic techniques and capabilities to treat sepsis effectively [53-55].
Addressing safety concerns related to magnetic particle-based blood-cleansing technology requires conducting a phase I clinical trial focused on assessing the biocompatibility of the particles. As magnetic particles reach the nano-scale range, the effectiveness of magnetic removal becomes challenging, and cytotoxicity increases. Long-term exposure studies have shown that intravenous administration of carbon-coated magnetic particles may not pose hazards. To meet the unmet needs in this field, future research should explore alternative approaches involving magnetic particles decorated with various functional features [56-58]. It is crucial to efficiently detect any remaining magnetic nanoparticles in the bloodstream after blood-cleansing treatment, ensuring that only a minimal amount of nanoparticles return to the patients. Existing tools for quantifying magnetic nanoparticles or measuring the magnetic susceptibility of whole blood require specialized equipment not commonly found in standard research laboratories. Researchers have developed microfluidic systems and detection methods to measure the concentrations of magnetic particles and detect extremely low levels in complex fluids, providing potential solutions for monitoring nanoparticle levels in conjunction with extracorporeal devices [59,60].
While the main focus of extracorporeal blood treatment methods has been reducing endotoxin or cytokine levels in septic patients, eradicating viral particles has received limited attention. Although the opsonin molecule FcMBL has been shown to bind various viral particles, there is currently no evidence of the effective elimination of viruses from human blood [61,62]. Further investigation is needed to determine the effectiveness of extracorporeal devices in reducing viral particle concentrations in the bloodstream. Animal models, particularly rodents, have been commonly used in in vivo experiments, such as Cecal Ligation and Puncture (CLP) and intravenous or intraperitoneal infection studies. However, these animal models cannot accurately predict human therapeutic outcomes. Before progressing to clinical trials, evaluating the effectiveness of developed technologies in larger animal models, such as swine, rabbits, or baboons, is essential [63,64]. Despite limitations in accurately replicating human septic pathophysiology and pharmacokinetics, developing extracorporeal blood treatment devices has been feasible using sepsis animal models, primarily focusing on pathogen level reduction, which may have comparable efficacy to human blood depletion.
Development of new strategies
Emerging evidence has shown that multiple strategies that proved effective in preclinical or phase II clinical trials have failed at the bigger multi-centre trial stage. Increasing appreciation of the different phenotypes in sepsis syndrome and post hoc analyses of the trial data indicating outcome benefits in specific subsets [65,66] suggest that these old agents should be revisited, particularly with the advent of diagnostics enabling fast subset identification.
Conclusion
Sepsis remains a significant global health challenge with high mortality rates and limited effective treatments, exacerbated by multidrug-resistant bacteria. Interdisciplinary research, particularly involving nanotechnology, holds promise for advancing extracorporeal treatments and enhancing therapeutic efficacy. Addressing the complexity of sepsis requires developing novel biomarkers, optimizing blood-cleansing devices, and exploring post-sepsis syndrome impacts. Collaborative efforts and innovative approaches are crucial to reducing sepsis mortality and improving survivors' quality of life.
Conflict of Interest
The authors declared the absence of conflict of interest.
About the Authors
Corresponding Author
Zhou Yu-ming
Department of Emergency, The Affiliated Ganzhou Hospital of Nanchang University, 341000 Ganzhou, Jiangxi Province, P.R. China
- Email:
- 18007079761@163.com
References
- Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, et al. (2021) Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Crit. Care. Med. 49: e1063-e1143.
[Crossref] [Google Scholar] [PubMed]
- Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, et al. (2017) Recognizing sepsis as a global health priority-A WHO resolution. N. Engl. J. Med. 377: 414-417.
[Crossref] [Google Scholar] [PubMed]
- Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, et al. (2020) Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the global burden of disease study. Lancet. 395: 200-211.
[Crossref] [Google Scholar] [PubMed]
- Yang Y, Ding Y, Fan B, Wang Y, Mao Z, et al. (2020) Inflammation-targeting polymeric nanoparticles deliver sparfloxacin and tacrolimus for combating acute lung sepsis. J. Control. Release. 321: 463-474.
[Crossref] [Google Scholar] [PubMed]
- Roberts JA, de Waele JJ, Dimopoulos G, Koulenti D, Martin C, et al. (2012) DALI: Defining Antibiotic Levels in Intensive care unit patients: A multi-centre point of prevalence study to determine whether contemporary antibiotic dosing for critically ill patients is therapeutic. BMC. Infect. Dis. 12: 1-7.
[Crossref] [Google Scholar] [PubMed]
- Roberts JA, Joynt GM, Lee A, Choi G, Bellomo R, et al. (2021) The effect of renal replacement therapy and antibiotic dose on antibiotic concentrations in critically ill patients: Data from the multinational sampling antibiotics in renal replacement therapy study. Clin. Infect. Dis. 72: 1369-1378.
[Crossref] [Google Scholar] [PubMed]
- Tacconelli E, Cataldo MA, Mutters NT, Carrara E, Bartoloni A, et al. (2019) Role of place of acquisition and inappropriate empirical antibiotic therapy on the outcome of extended-spectrum β-lactamase-producing Enterobacteriaceae infections. Int. J. Antimicrob. Agents. 54: 49-54.
[Crossref] [Google Scholar] [PubMed]
- Kern WV and Rieg S (2020) Burden of bacterial bloodstream infection-A brief update on epidemiology and significance of multidrug-resistant pathogens. Clin. Microbiol. Infect. 26: 151-157.
[Crossref] [Google Scholar] [PubMed]
- Reynolds D, Burnham JP, Guillamet CV, McCabe M, Yuenger V, et al. (2022) The threat of multidrug-resistant/extensively drug-resistant gram-negative respiratory infections: Another pandemic. Eur. Respir. Rev. 31.
[Crossref] [Google Scholar] [PubMed]
- Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell. 140: 805-820.
[Crossref] [Google Scholar] [PubMed]
- Chan JK, Roth J, Oppenheim JJ, Tracey KJ, Vogl T, et al. (2012) Alarmins: Awaiting a clinical response. J. Clin. Invest. 122: 2711-2719.
[Crossref] [Google Scholar] [PubMed]
- Pisetsky DS, Gauley J and Ullal AJ (2011) HMGB1 and microparticles as mediators of the immune response to cell death. Antioxid. Redox. Signal. 15: 2209-2219.
[Crossref] [Google Scholar] [PubMed]
- Dendoncker K, Libert C (2017) Glucocorticoid resistance as a major drive in sepsis pathology. Cytokine. Growth. Factor. Rev. 35: 85-96. [Crossref]
[Google Scholar] [PubMed]
- Angus DC, Van der Poll T (2013) Severe sepsis and septic shock. N. Engl. J. Med. 369: 840-851.
[Crossref] [Google Scholar] [PubMed]
- Pfalzgraff A and Weindl G (2019) Intracellular lipopolysaccharide sensing as a potential therapeutic target for sepsis. Trends. Pharmacol. Sci. 40: 187-197.
[Crossref] [Google Scholar] [PubMed]
- Perez-Hernandez EG, Delgado-Coello B, Luna-Reyes I and Mas-Oliva J (2021) New insights into lipopolysaccharide inactivation mechanisms in sepsis. Biomed. Pharmacother. 141: 111890.
[Crossref] [Google Scholar] [PubMed]
- Kang JS, Jeon YJ, Park SK, Yang KH, Kim HM (2004) Protection against lipopolysaccharide-induced sepsis and inhibition of interleukin-1β and prostaglandin E2 synthesis by silymarin. Biochem. Pharmacol. 67: 175-181.
[Crossref] [Google Scholar] [PubMed]
- Anderson ST, Commins S, Moynagh PN and Coogan AN (2015) Lipopolysaccharide-induced sepsis induces long-lasting affective changes in the mouse. Brain. Behav. Immun. 43: 98-109.
[Crossref] [Google Scholar] [PubMed]
- Tunctan B, Uluda O, Altu S (1998) Effects of nitric oxide synthase inhibition in lipopolysaccharide-induced sepsis in mice. Pharmacol. Res. 38: 405-411.
[Crossref] [Google Scholar] [PubMed]
- Ni J, Zhao Y, Su J, Liu Z, Fang S, et al. (2020) Toddalolactone protects lipopolysaccharide-induced sepsis and attenuates lipopolysaccharide-induced inflammatory response by modulating HMGB1-NF-κB translocation. Front. Pharmacol. 11: 109.
[Crossref] [Google Scholar] [PubMed]
- Liu Z, Chen X, Ma W, Gao Y, Yao Y, et al. (2022) Suppression of lipopolysaccharide-induced sepsis by tetrahedral framework nucleic acid loaded with quercetin. Adv. Funct. Mater. 32: 2204587.
- Opal SM and Patrozou E (2009) Translational research in the development of novel sepsis therapeutics: Logical deductive reasoning or mission impossible? Crit. Care. Med. 37: S10-S15.
[Crossref] [Google Scholar] [PubMed]
- Kim BY, Rutka JT and Chan WC (2010) Nanomedicine. N. Engl. J. Med. 363: 2434-2443.
[Crossref] [Google Scholar] [PubMed]
- Halwani AA (2022) Development of pharmaceutical nanomedicines: From the bench to the market. Pharmaceutics. 14: 106.
[Crossref] [Google Scholar] [PubMed]
- Demetzos C and Demetzos C (2016) Application of nanotechnology in imaging and diagnostics. Pharm. Nanotechnol: Fundamen. Prac. Appli. 65-75.
- Herrmann IK (2015) How nanotechnology-enabled concepts could contribute to the prevention, diagnosis and therapy of bacterial infections. Crit. Care. 19: 239.
[Crossref] [Google Scholar] [PubMed]
- Etheridge ML, Campbell SA, Erdman AG, Haynes CL, Wolf SM, et al. (2013) The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomed: Nanotechnol, Biol. Med. 9: 1-4.
[Crossref] [Google Scholar] [PubMed]
- Handa M, Sharma A, Verma RK and Shukla R (2019) Polycaprolactone based nano-carrier for co-administration of moxifloxacin and rutin and its in-vitro evaluation for sepsis. J. Drug. Deliv. Technol. 54: 101286.
[Crossref]
- Hou X, Zhang X, Zhao W, Zeng C, Deng B, et al. (2020) Vitamin lipid nanoparticles enable adoptive macrophage transfer for the treatment of multidrug-resistant bacterial sepsis. Nat. Nanotechnol. 15: 41-46.
[Crossref] [Google Scholar] [PubMed]
- Parlato M and Cavaillon JM (2015) Host response biomarkers in the diagnosis of sepsis: A general overview. Methods. Mol. Biol. 149-211.
[Crossref] [Google Scholar] [PubMed]
- Parlato M, Philippart F, Rouquette A, Moucadel V, Puchois V, et al. (2018) Circulating biomarkers may be unable to detect infection at the early phase of sepsis in ICU patients: The CAPTAIN prospective multicenter cohort study. Intensive. Care. Med. 44:1061-70.
[Crossref] [Google Scholar] [PubMed]
- Mickiewicz B, Tam P, Jenne CN, Leger C, Wong J, et al. (2015) Integration of metabolic and inflammatory mediator profiles as a potential prognostic approach for septic shock in the intensive care unit. Crit. Care. 19: 1-2.
[Crossref] [Google Scholar] [PubMed]
- Sweeney TE, Azad TD, Donato M, Haynes WA, Perumal TM, et al. (2018) Unsupervised analysis of transcriptomics in bacterial sepsis across multiple datasets reveals three robust clusters. Crit. Care. Med. 46: 915-925.
[Crossref] [Google Scholar] [PubMed]
- Ellett F, Jorgensen J, Marand AL, Liu YM, Martinez MM, et al. Diagnosis of sepsis from a drop of blood by measurement of spontaneous neutrophil motility in a microfluidic assay. Nat. Biomed. Eng. 2: 207-214.
[Crossref] [Google Scholar] [PubMed]
- Oved K, Cohen A, Boico O, Navon R, Friedman T, et al. (2015) A novel host-proteome signature for distinguishing between acute bacterial and viral infections. PloS. One. 10(3):e0120012.
[Crossref] [Google Scholar] [PubMed]
- Miller III RR, Lopansri BK, Burke JP, Levy M, Opal S, et al. (2018) Validation of a host response assay, SeptiCyte LAB, for discriminating sepsis from systemic inflammatory response syndrome in the ICU. Am. J. Respir. Crit. Care. Med. 198: 903-913.
[Crossref] [Google Scholar] [PubMed]
- Chung AJ (2019) A minireview on inertial microfluidics fundamentals: Inertial particle focusing and secondary flow. Bio. Chip. J. 13: 53-63.
- Hur J, Park I, Lim KM, Doh J, Cho SG, et al. (2020) Microfluidic cell stretching for highly effective gene delivery into hard-to-transfect primary cells. ACS nano. 14: 15094-15106.
[Crossref] [Google Scholar] [PubMed]
- Hur J and Chung AJ (2021) Microfluidic and nanofluidic intracellular delivery. Adv. Sci (Weinh). 8: 2004595.
[Crossref] [Google Scholar] [PubMed]
- Xu X, Huang X, Sun J, Wang R, Yao J, et al. (2021) Recent progress of inertial microfluidic-based cell separation. Analyst. 146: 7070-7086.
[Crossref] [Google Scholar] [PubMed]
- Zhao Q, Yuan D, Zhang J, Li W (2020) A review of secondary flow in inertial microfluidics. Micromachines. 11: 461.
[Crossref] [Google Scholar] [PubMed]
- Tang W, Zhu S, Jiang D, Zhu L, Yang J, et al. (2020) Channel innovations for inertial microfluidics. Lab Chip. 20: 3485-3502.
[Crossref] [Google Scholar] [PubMed]
- Alseed MM, Rahmani Dabbagh S, Zhao P, Ozcan O, Tasoglu S (2021) Portable magnetic levitation technologies. Advan. Opt. Technol. 10: 109-121.
- Koo D and So H (2022) Facile microfabrication of three dimensional-patterned micromixers using additive manufacturing technology. Sci. Rep. 12: 6346.
[Crossref] [Google Scholar] [PubMed]
- Schemberg J, Abbassi AE, Lindenbauer A, Chen LY, Grodrian A, et al. (2022) Synthesis of biocompatible superparamagnetic iron oxide nanoparticles (SPION) under different microfluidic regimes. ACS. Appl. Mater. Interfaces. 14: 48011-48028.
[Crossref] [Google Scholar] [PubMed]
- Rodriguez-Mateos P, Ngamsom B, Dyer CE, Iles A, Pamme N (2021) Inertial focusing of microparticles, bacteria, and blood in serpentine glass channels. Electrophoresis. 42: 2246-2255.
[Crossref] [Google Scholar] [PubMed]
- Liu W, Wang R, Vedarethinam V, Huang L, Qian K (2022) Advanced materials for precise detection and antibiotic-free inhibition of bacteria. Mater. Today. Adv. 13: 100204.
- Jalilvand E, Shamloo A and Gangaraj MH (2022) Computational study of an integrated microfluidic device for active separation of RBCs and cell lysis. Chem. Eng. Process: Process. Intensif. 174: 108891.
- Li FC, Liu T, Zhang JY, Shuang S, Wang Q, et al. (2019) Materials today advances. Sort. 50: 200.
- Omar C. Microfluidic platform for lysis, sorting and detection: Towards a rapid diagnosis of septicemia. Doctoral dissertation, University Paris-Saclay.
- Dela Cruz P, Hung CM, Marsden D, Prasanna N, Chu E, et al. Correlational analysis of eaa with other inflammatory markers in critically ill SARS-CoV-2 Patients.
- Lentini P, de Cal M, Clementi A, D′ Angelo A, Ronco C (2012) Sepsis and AKI in ICU patients: The role of plasma biomarkers. Crit. Care. Res. Pract. 2012: 856401.
[Crossref] [Google Scholar] [PubMed]
- Ronco C, Cruz D, Nalesso F and Piccinni P (2010) Extracorporeal endotoxin removal in sepsis. In Anaesthesia, pharmacology, intensive care and emergency medicine APICE: Proceedings of the 23rd postgraduate course in critical care medicine catania, Italy-November 5-7, 2011:243-248. Springer Milan.
- Ronco C, Artigas A and Antonelli M. B-Based Hemoperfusion for the Treatment of Endotoxic Shock.
- Dellepiane S, Marengo M and Cantaluppi V (2016) Detrimental cross-talk between sepsis and acute kidney injury: New pathogenic mechanisms, early biomarkers and targeted therapies. Crit. Care. 20: 1-1.
[Crossref] [Google Scholar] [PubMed]
- Macdonald SP, Stone SF, Neil CL, van Eeden PE, Fatovich DM, et al. (2014) Sustained elevation of resistin, NGAL and IL-8 are associated with severe sepsis/septic shock in the emergency department. PLoS. One. 9: e110678.
[Crossref] [Google Scholar] [PubMed]
- Hsu YC and Hsu CW (2019) Septic acute kidney injury patients in emergency department: The risk factors and its correlation to serum lactate. Am. J. Emerg. Med. 37(2):204-208.
[Crossref] [Google Scholar] [PubMed]
- Kim S, Kim HJ, Ahn HS, Song JY, Um TH, et al. (2016) Is plasma neutrophil gelatinase-associated lipocalin a predictive biomarker for acute kidney injury in sepsis patients? A systematic review and meta-analysis. J. Crit. Care. 33: 213-223.
[Crossref] [Google Scholar] [PubMed]
- Peng Q, Zhang L, Ai Y and Zhang L (2014) Epidemiology of acute kidney injury in intensive care septic patients based on the KDIGO guidelines. Chin. Med. J. 127: 1820-1826.
[Google Scholar] [PubMed]
- Zhu Y, Wei SW, Ding A, Zhu WP, Mai MF, et al. (2020) The long noncoding RNA ANRIL promotes cell apoptosis in lipopolysaccharide-induced acute kidney injury mediated by the TLR4/nuclear factor-kappa B pathway. Kidney. Blood. Press. Res. 45: 209-221.
[Crossref] [Google Scholar] [PubMed]
- Didar TF, Cartwright MJ, Rottman M, Graveline AR, Gamini N, et al. (2015) Improved treatment of systemic blood infections using antibiotics with extracorporeal opsonin hemoadsorption. Biomaterials. 67: 382-92.
[Crossref] [Google Scholar] [PubMed]
- Yousefi H, Su HM, Imani SM, Alkhaldi K, M. Filipe CD, et al. (2019) Intelligent food packaging: A review of smart sensing technologies for monitoring food quality. ACS. Sensors. 4: 808-821.
[Crossref] [Google Scholar] [PubMed]
- Monard C, Rimmele T, Ronco C (2019) Extracorporeal blood purification therapies for sepsis. Blood. Purif. 47: 2-15.
[Crossref] [Google Scholar] [PubMed]
- Badv M, Bayat F, Weitz JI and Didar TF (2020) Single and multi-functional coating strategies for enhancing the biocompatibility and tissue integration of blood-contacting medical implants. Biomaterials. 258: 120291.
[Crossref] [Google Scholar] [PubMed]
- Calfee CS, Delucchi KL, Sinha P, Matthay MA, Hackett J, et al. (2018) Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: Secondary analysis of a randomised controlled trial. The Lancet. Respir. Med. 6: 691-698.
[Crossref] [Google Scholar] [PubMed]
- Antcliffe DB, Burnham KL, Al-Beidh F, Santhakumaran S, Brett SJ, et al. (2019) Transcriptomic signatures in sepsis and a differential response to steroids. From the VANISH randomized trial. Am. J. Respir. Crit. Care. Med. 199: 980-986.
[Crossref] [Google Scholar] [PubMed]
Keywords:
Download:
Full PDF- Share This