Noonan syndrome: Discovering new mutations?
Received: November 22, 2024
Accepted: November 25, 2024
Published: December 30, 2024
Genet.Mol.Res. 23(4):
Keywords
Noonan syndrome; RASopathies/ Síndrome de Noonan; RASopatias
Introduction
Noonan Syndrome (NS) is one of the most common genetic conditions. The incidence of NS is estimated as 1 in 1,000 to 1 in 2,500 births, so it is still a relatively rare condition. The severity of NS is the same in males and females. The main features are congenital heart defects, short stature and characteristic facial features. Early motor delay associated with hypotonia is not necessarily associated with later learning difficulty, and most adults with NS are able to lead independent autonomous lives [1].
Since 2001, it is known that mutations in genes that encode proteins involved in the intracellular RAS-MAPK (Mitogen Activated Protein Kinases) signaling pathway are responsible for NS and other genetic disorders with similar phenotypes, such as costello syndrome, cardiofaciocutaneous syndrome, Noonan Syndrome with Multiple Lentigines (NS-ML, formerly known as LEOPARD syndrome) or neurofibromatosis type 1.
No consensus clinical diagnostic criteria for Noonan syndrome have been published. Diagnostic scoring systems, most recently published in van der Burgt and embedded in the management guidelines developed by Dyscerne in the United Kingdom (Noonan Syndrome Guideline Development Group 2010), have been proposed but have not been used extensively in North America (Table 1) [2,3].
| S.no | Feature | A=Major | B=Minor | ||
|---|---|---|---|---|---|
| 1 | Facial | Typical face (Facial features of NS vary over time and may have only subtle differences. Expert assessment is therefore Required. | Suggestive face. | ||
| 2 | Cardiac | Pulmonary valve stenosis and/or hypertrophic cardiomyopathy. | Other cardiac defect. | ||
| 3 | Height | < 3th centile | <10th centile. | ||
| 4 | Chest wall | Pectus carinatum/excavatum. | Broad thorax. | ||
| 5 | Family History | First degree relative with definite NS. | First degree relative suggestive of NS. | ||
| 6 | Other | Mild developmental delay, cryptorchidism AND lymphatic dysplasia. | Mild developmental delay, cryptorchidism, OR lymphatic dysplasia. | ||
| Definitive NS: | |||||
| Criterion 1A+ | Criterion 1B+ | ||||
| One of 2A-6A | Two of 2B-6B | Two of 2A-6A | Three of 2B-6B | ||
Table 1. Diagnostic features of NS.
Currently, mutation testing will prove a diagnosis of Noonan syndrome in 70% of cases; in 30% of cases the responsible gene remains unknown. Given the number of different genes where mutations can cause NS, the indication of making a sequence of genes should be decided by a clinical geneticist [1].
Molecular genetic testing approaches can include a combination of gene-targeted testing (multi-gene panel) and comprehensive genomic testing (exome sequencing or genome sequencing) depending on the phenotype [3].
The molecular diagnosis of NS is established in a pro-band with suggestive findings and a heterozygous pathogenic (or likely pathogenic) variant in one of the genes listed in Table 2 or bi-allelic pathogenic variants in LZTR1 [3].
| Gene | Proportion of NS attributed to pathogenic variants in gene | Proportion of pro-bands with a pathogenic variant detected by method | |
|---|---|---|---|
| Sequence analysis | Gene- targeted deletion/duplication analysis | ||
| BRAF | 100% | Unknown | |
| KRAS | 100% | Unknown | |
| LZTR1 | 100% | Unknown | |
| MAP2K1 | 100% | Unknown | |
| MRAS | 100% | Unknown | |
| NRAS | 100% | Unknown | |
| PTPN11 | Nearly 100% | Rare duplication, diagnosis of NS questioned | |
| RAF1 | Nearly 100% | 1 reported case of duplication, diagnosis of NS questioned; 1 reported case of a deletion | |
| RASA2 | 100% | Unknown | |
| RIT1 | 100% | Unknown | |
| RRAS2 | 100% | Unknown | |
| SOS1 | 100% | Unknown | |
| SOS2 | 100% | Unknown | |
Table 2. Molecular genetic testing used in Noonan syndrome.
Case Presentation
A 50-year-old white male was brought by his mother to the emergency services in Albacete (Spain) due to the presence of speech disturbances and drowsiness in the past four or five days. He was supposed to have fallen to the floor, but he was not seen by any witness, so they were not able to say if there was any chance of him having had his head beaten. During the anamnesis, the patient denied any type of symptoms: Dyspnea, cough, chest pain, abdominal pain, voiding clinic, fever but he was having an incoherent speech and quite a childish behavior.
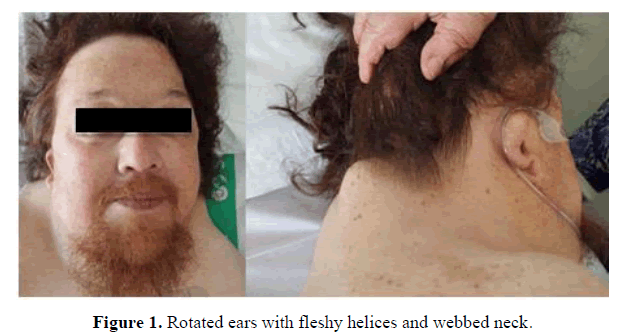
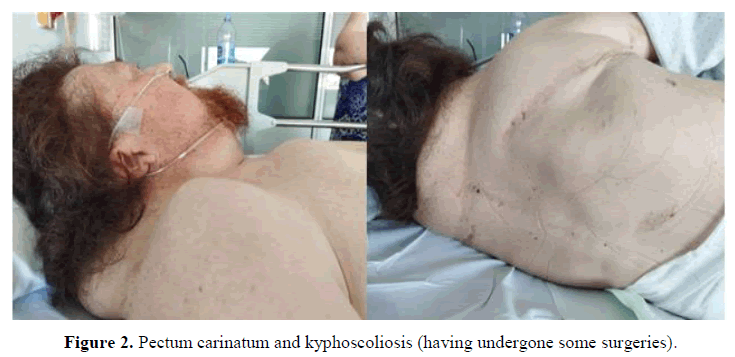
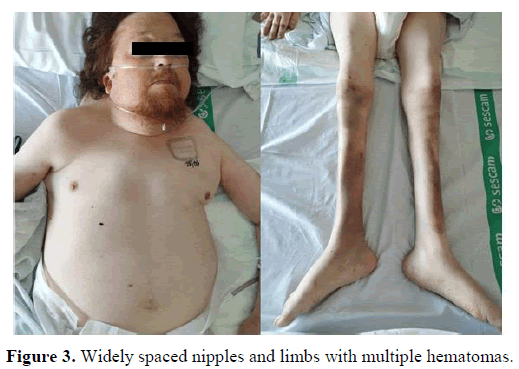
In the physical examination, some clinical characteristics were identified which could suggest the existence of a genetic syndrome, such as: Short stature, low set and posteriorly rotated ears with fleshy helices, pterygium colli, superior pectus carinatum and inferior pectus excavatum, widely spaced nipples and kypho-scoliosis (having undergone some surgeries). The cardiopulmonary auscultation and abdominal palpation did not show any alteration. The limbs had multiple hematomas, professedly with no previous trauma. There were no edemas or signs of thrombosis.
Looking for information in his personal medical history, the following findings seemed relevant: Diagnosis of hypertension, chronic kidney disease, rheumatoid arthritis, past infection with cytomegalovirus in 2012 and cardiac anomalies diagnosed years before, some of them treated: Aortic valve disease, severe pulmonary valvestenosis, hypertrophic cardiomyopathy, patent foramen ovale, and silent coronary disease (treated with two drug-eluting stents) (Figures 1-3).
His father had died a few years ago, whose phenotype was quite similar to his (comparing with photos). His mother had a phenotype apparently not compatible with a genetic syndrome. He did not have any brothers or sisters.
Several blood tests were taken and hypercalcemia with serum calcium of 13,6 mg/dL (reference values between 8.5 to 10.2) was detected. Also, high figures of para-thormone were detected in previous analysis with figures around 300-400 pg/mL (reference values 10 to 55). For this reason, a neck ultrasound was performed in order to detect any cause of primary hyperparathyroidism. The findings showed two defined nodes, heterogeneous in consistency measuring 2.4 cm ⨯ 0.9 cm. The rest of parameters of the blood tests were normal, except from the presence of anemia, with figures of hemoglobin around 10.5 gr/dL (reference values for men 13.2 to 16.6), with no symptoms of hemorrhages.
Otherwise, an electrocardiogram, a chest x-ray and a brain CT were performed, with no significant alterations. A little increase of the volume of the ventricular system of brain was described, probably secondary to cortical- subcortical retraction.
During the admission, the figures of calcemia improved with specific treatment, achieving normal values before the patient was discharged. Nevertheless, due to the personal medical history and also because of the striking phenotype of the patient, we proposed him to undergo a genetic study and he accepted. Then, he left the hospital pending of some tests: The genetic study and also a thyroid scan.
Genetic study
A massive sequencing study was carried out in order to identify the genomic variants in the genes A2ML1, BRAF, CBL, HRAS, KRAS, LZTR1, MAP2K1, MAP2K2, MAPK1, NF1, NRAS, PTPN11, RAF1, RASA1, RASA2, RIT1, RRAS2, SHOC2, SOS1, SOS2 and SPRED1 [4].
Biological/clinical interpretation of the variant detected: PTPN11 c.774G>C; p(Glu258Asp). The presence of variants in PTPN11 is associated with an autosomal dominant inheritance pattern to Noonan syndrome 1 (MIM#163950), leopard syndrome 1 (MIM#151100), among others.
The variant identified in heterozygosity in the patient's sample is variant of change of direction that had not been registered in the population databases (gnomAD) nor had it been previously described in the literature or in the databases consulted associated with a specific phenotype. But, it should be noted that two other missense changes involving the same amino acid had been classified as pathogenic/probably pathogenic (p.Glu258Lys and p.Glu258Asp; Variation ID: 982429 and 44613, respectively) associated with Noonan syndrome, giving rise to one of them the same change at the protein level as that identified in the patient (c.774G>T, p.Glu258Asp; Variation ID: 44613). Also, at the beginning of the PTP domain where this change was located, in the 10 adjacent amino acids had been described 9 pathogenic/ probably pathogenic variants, some of them having been described in patients with Noonan syndrome. Additionally, the in-silico analysis with most of the prediction programs used and the CADD value (16.19) were compatible with the variant being deleterious.
Based on the fact that it is a variant of misdirection absent in control population that the prediction systems suggest a deleterious effect, which is located in a hot spot where other variants of misdirection had been described as pathogenic/probably pathogenic, one of them that gives rise to the same change at the level of the protein that had been classified as pathogenic/probably pathogenic, is categorized as a probably pathogenic variant.
The degree of association of the variant identified in the PTPN11 gene with the clinic of the patient studied should be evaluated in the clinical context of the patient. If it is established that there is a possible phenotype/genotype association, the recommendation is to evaluate by sanger sequencing the pattern of family segregation of the identified variant, especially in its parents to try to determine if it is an inherited or de novo event.
Discussion
While the genetic study was been carrying out, the patient was admitted to the hospital again a few weeks later because of a SARS-CoV-2 pneumonia with respiratory insufficiency, ending up with a fatal outcome. This was the reason why many tests did not get to carry out.
Approximately two months after his demise and having received the results of the genetic study, our team (formed by geneticists and internal medicine professionals) contacted with his mother in order to propose her to do an ampliation of the study due to the fact that it was unknown for the team if the identified variant was caused by an inherited mutation or by “de novo event”. Sadly, she refused the proposal and it could not be possible to determine the nature of it. The patient gave us his consent to post any scientific discoveries with his photos before his death.
So that the genetic categorization as “probably pathogenic” of the variant PTPN11 c.774G>C; p(Glu258Asp), compatible with Noonan´s syndrome, could change if segregation, functional studies or new cases with this change and a phenotype similar to that of the patient are described in the future.
Besides, most studies have found a higher incidence of autoimmune thyroiditis compared to the general population, (2) but not primary hyperparathyroidism have been described in the literature, so another question we have asked is if there could be any relation between the suspected diagnosis of our patient and the Noonan syndrome.
Conclusion
Noonan syndrome is a clinical entity with substantial heterogeneity in its clinical features and underlying genetics, requiring a global evaluation and multidisciplinary approach to its management and regular follow-up. In recent decades, advances in gene sequencing techniques have allowed the identification of the genetic cause of NS in most patients.
Hopefully, these studies will allow the detection of new variants which could explain the presence of Noonan syndrome, in order to develop gene-targeted therapies to treat NS and other RASopathies.
Conflict of Interests
This work has not received any specific support from public sector agencies, commercial sector or non-profit entities. The authors declare they have no conflict of interest.
About the Authors
Corresponding Author
Marta Guzmán Pérez
Department of Internal Medicine, Albacete University Hospital, 02006 Albacete, Spain
- Email:
- martaguzpe661@gmail.com
References
- Noonan Syndrome Guideline Development Group (2010) Management of Noonan syndrome. A clinical guideline. University of Manchester: Dyscerne.
- Carcavilla A, Suarez-Ortega L, Sanchez AR, Gonzalez-Casado I, Ramon-Krauel, et al. (2020) Noonan syndrome: Genetic, clinical, and therapeutic options update. Ann. Pediatr. 93: 61-e1.
- Roberts AE (2001) Noonan syndrome. In: Adam MP, Everman DB, Mirzaa GM, et al., Editors. GeneReviews®. Seattle (WA): University of Washington, Seattle. 1993-2022.
[Google Scholar] [PubMed]
- Management of Noonan syndrome
Keywords:
Download:
Full PDF- Share This