The effect of acetate Ringer's solution on inflammatory factors and NF-テηステつコB signal pathway in the liver of hemorrhagic shock rats
Received: March 27, 2018
Accepted: April 17, 2018
Published: April 20, 2018
Genet.Mol.Res. 17(2): gmr16039906
DOI: 10.4238/gmr16039906
Abstract
Purpose: To investigate the effect of resuscitation with acetated Ringer's solution during hemorrhagic shock (HS) on inflammatory factors and nuclear factor κB (NF-κB) signaling pathway in liver tissue. Methods: In this study, a rat HS model was established. HS rats received acetated Ringer's solution, lactated Ringer's solution and saline for fluid resuscitation. Inflammatory factors (TNF-α, IL-4 and IL-10) for the evaluation of three kinds of resuscitation mfluid anti-inflammatory effect. Expression of proteins in NF-κB pathway was evaluated to elucidate the mechanism of acetated Ringer's solution in preventing HS-induced liver injury. Results: Although HS upregulated inflammatory response in liver tissue, acetated Ringer's solution t-treatment can inhibit the inflammation and improve liver injury. We also found that acetated ringer’s solution treatment attenuated HS-induced liver injury in NF-κB pathway. Conclusion: Acetated Ringer's solution may improve HS-induced liver injury. The mechanism of its action may be by inhibiting the NF-κB pathway.
Introduction
Hemorrhagic shock (HS) is the main cause of death and disability in the world, which increases the burden of limited medical resources (Cocchi et al., 2007). The most important and basic measure to treat hemorrhagic shocks fluid resuscitation. However, we often find in clinical practice that correcting the tissue's low blood perfusion often fails to correct shock. This is because the pathophysiological changes in shock have not only hemodynamic changes but also inflammatory reactions. HS pathophysiology complex, which involves the systemic inflammatory response and pathological changes, such as: hypovolemia, hypoxemia, microcirculation and oxidative stress (Angele et al., 2008). Multiple organ dysfunction syndrome (MODS) caused by systemic inflammatory response syndrome (SIRS) is one of the major causes of HS and death (Chen et al., 2013). Complications of HS are thought to be associated with abnormalities in the immune system and activation of deleterious factors, which may be manifested as immunosuppression or proinflammatory state (Fukudome et al., 2012). Nuclear factor-κB (NF-κB) activates the inflammatory cascade after HS and accelerates the release of pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α) (EI-Tahan et al., 2016). Liver injury is an important part of MODS resulting from HS (Liu et al., 2017). Therefore, it is very important to study the inhibition of NF-κB pathway in the liver of HS rats. Interestingly, our previous study (Cai et al., 2016) s-howed that as a new balanced salt solution, acetated Ringer's solution compared to lactated Ringer's solution can reduce the body's inflammatory response. However, whether acetated Ringer's solution can reduce the release of inflammatory mediators in HS by affecting NF-κB pathway has not been reported. In this study, we observed the changes of TNF-α, IL-4 and IL-10 mRNA in liver tissue and the expression of p65 (Ser536) phosphorylation and p65 (Lys310) acetyl-ation to explore the role of acetated Ringer's solution in the inhibition of possible mechanism of HS Inflammatory reaction.
Materials and Methods
Experimental materials and grouping
Thirty-two healthy Sprague-Dawey (SD) rats, SPF (Specific Pathogen Free) grade, male and fe-male, body weight (300 ± 20) g, provided by Shanghai Jiesijie Experimental Animal Company. Experimental animals were feeded for 7 days at room temperature (22 ± 1°C) and humidity (55-45)% with 12 hours light/dark cycle. The rats were randomly divided into four groups: not resuscitation group (CR group, n=8), normal saline resuscitation group (NR group, n=8), lactated Ringer's solution resuscitation group (LR group, n=8) and acetated Ringer's solution r-resuscitation group (AR group, n=8).
Animal model
Rats were anesthetized by intraperitoneal injection of 4% chloral hydrate (1 mL/100 g). After successful anesthesia, the limbs of rats were fixed on a sterile operating table and the operating room temperature was maintained at 25°C. After successful anesthesia, rats were given groin area on both sides skin preparation, disinfection, shop towels. After incision with incandescent lamp irradiation, dissecting the femoral vein and femoral artery. Mean arterial pressure(MAP) is continuously monitored using the MedLab-U/4C501H BioSignal Acquisition Processing System. After catheterization, rats adapted to 20 min. Blood was slowly released from the fem-oral artery at a rate of 2 mL/3 min with a 1 mL syringe until MAP reached 40-45 mmHg. MAP (40-45 mmHg) was maintained for 60 min by slowly returning autologous blood or a s-mall amount of bleeding. Rats in NR, LR and AR groups were resuscitated with normal saline, lactated Ringer's solution and acetated Ringer's solution respectively after 30 min of shock maintenance (MAP was completed in 30min), and MAP was increased to 80 mmHg to correct shock. After recovery observed 4 hours to take liver tissue. No resuscitation was observed in CR group (liver tissue was observed 4 hours after induction of shock).
Observation indicators and methods
Real-time quantitative PCR was used to detect the relative expression of TNF-α, IL-4 and IL-10 mRNA in liver tissue. The relative expression of p65 (Ser536) phosphorylated protein and P65 (Lys310) acetylated protein in liver tissue were detected by Western Blot.
Real-time quantitative PCR
RNA of liver tissue was extracted according to Trizol kit (invitrogen). The configuration of the reaction liquid in reverse transcription was according to the invitrogen reverse transcription kit superscript III, inactivation at 6°C for 10 min to remove DNA, add primer mix 42°C reverse transcription 1 hour. After taking out, it was placed at 85°C and reacted for 10 minutes to inactivate reverse transcriptase placed at -20°C after reaction. After the cDNA was diluted 10 times, the primer reaction system was added as a PCR template. On the ABI 7900 qPCR instrument, pre-denatured at 95°C for 2 min, denatured at 94°C for 20 s, annealed at 60°C for 20 s, extended at 72°C for 30 s and performed 40 cycles. The result was finally obtained by reading the Ct (cycle-threshold) value and calculating 2-ΔΔCt. The upstream primer of TNF-α was 5'-ATG GGC TCC CTC TCA TCA GT -3 ', the downstream primer was 5'-GCT TGG TGG TTT GCT ACG AC -3' and the product length was 106 bp. The upstream primer of IL-4 was: 5'-CTT GCT GTC ACC CTG TTC TG-3 ', and the downstream primers were: 5'-CTC CGT GGT GTT CCT TGT TG-3' with a product length of 174 bp. The upstream primer of IL-10 was: 5'-TGC GAC GCT GTC ATC GAT TT-3 ', and the downstream primers were: 5'-GTA GAT GCC GGG TGG TTC AA -3', product length 186 bp. GAPDH upstream primer 5'- GTT ACC AGG GCT GCC TTC TC-3 ', and the downstream primers were: 5'-GGG TTT CCC GTT GAT GAC C-3 'and the product length was 168 bp.
Western blot test
Taken frozen liver tissue, added the lysate for 1 min, centrifuged for 30 min, absorbed the supernatant was the total protein, and placed it at -80°C for using. Protein concentration detected with BCA kit. Proteins were separated by SDS-PAGE and transferred to PVDF membranes by transfer electrophoresis. After blocking with 5% skim milk powder for 2 hours at room temperature, the corresponding antibody I was added and incubated overnight at 4°C. And washed the membrane 3 times with BST, added alkaline phosphatase (AP) labeled antibody II, incubating for 1 h at room temperature, washed 3 times with TBST. After performing ECL chemi-luminescence, developing, fixing and photographing, optical densitometry of protein bands was performed using image analysis software Scion Image. The ratio of the target protein to the GAPDH gray value was calculated and statistically analyzed.
Statistical analysis
Results were analyzed by SPSS 22. 0. Data were expressed as mean ± standard deviation (x ± s). The comparison between groups using one-way ANOVA. Between the two groups using LSD-t test method analysis. P<0.5 indicates that the difference was statistically significant.
Results
Comparison initial of body weight and basal MAP between SD rats in each group
Each group had similar body weight and MAP. With mean ± standard deviation were expressed as: 286.75 ± 15.63 g vs. 102.35 ± 4. 17 mmHg, 284.38 ± 10.16 g vs. 102.85 ± 5.94 mmHg, 287. 12 ± 13.70 g vs. 106.25 ± 4.34 mmHg, 286.13 ± 17.75 g vs. 100.85 ± 7.20 mmHg. Take one-way ANOVA, there was no significant difference in body weight and MAP between the four groups (F=0.056, p>0.05 and F=1.345, p>0.05) (Table 1).
| Group | N | Weight (g) | MAP (mmHg) |
|---|---|---|---|
| CR Group | 8 | 286.75 ± 15.63 | 102.35 ± 4.17 |
| NR Group LR Group AR Group |
8 8 8 |
284.38 ± 10.16 287.12 ± 13.70 286.13 ± 17.75 |
102.85 ± 5.94 106.25 ± 4.34 100.85 ± 7.20 |
Table 1. One-way ANOVA analysis was used to estimate the difference between groups. Data were expressed as mean ± standard deviation
Acetated Ringer's solution treatment could reduce inflammation in HS rats
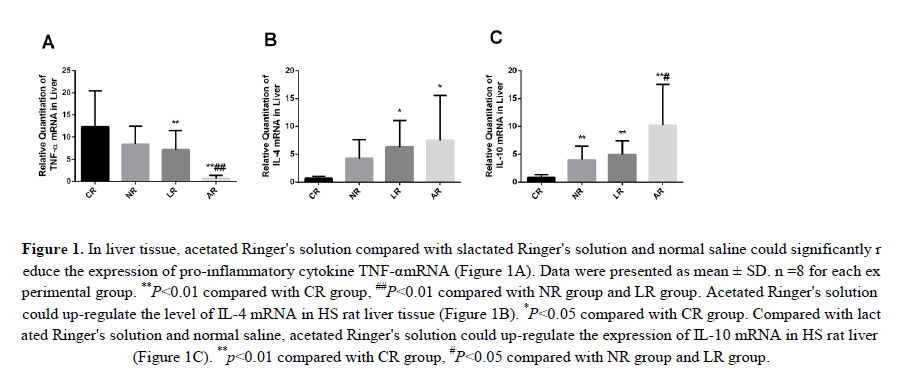
Associated with HS-induced organ damage was the inflammatory response. Therefore, we investigated the expression levels of pro-inflammatory cytokines TNF-α and IL-4, IL-10 in liver tis-sue to determine whether HS enhanced the inflammatory response and acetated Ringer's solution compared to lactated Ringer's solution and normal saline would significantly reduce these changes. We found that the relative expression of TNF-α in liver tissue was significantly upregulated in CR group, but decreased in NR, LR and AR groups (TNF-α expression in NR group was not significantly reduced compared with CR group. And compared with NR and LR groups, the expression of TNF-α was significantly decreased in AR group (Figure 1A). Interestingly, the levels of IL-4 and IL-10 in liver tissue were significantly increased in AR group compared with those in CR group. It is noteworthy that the expression level of L-10 in AR group is more pronounced in the liver tissue than in NR and LR groups (Figures 1B and 1C). The up-regulation of IL-4 and IL-10 anti-inflammatory factors can counteract the inflammatory reaction and balance the secretion of inflammatory cytokines. Our study showed that sodium acetated Ringer's solution not only reduced TNF-α level, but also increased the level of IL-4, IL-10 expression. This supports our hypothesis that acetated Ringer's solution has an anti-inflammatory effect.
Figure 1: In liver tissue, acetated Ringer's solution compared with slactated Ringer's solution and normal saline could significantly reduce the expression of pro-inflammatory cytokine TNF-αmRNA (Figure 1A). Data were presented as mean ± SD. n =8 for each experimental group. **P<0.01 compared with CR group, ##P<0.01 compared with NR group and LR group. Acetated Ringer's solution could up-regulate the level of IL-4 mRNA in HS rat liver tissue (Figure 1B). *P<0.05 compared with CR group. Compared with lactated Ringer's solution and normal saline, acetated Ringer's solution could up-regulate the expression of IL-10 mRNA in HS rat liver (Figure 1C). **p<0.01 compared with CR group, #P<0.05 compared with NR group and LR group.
Acetated Ringer's solution treatment inhibited NF-κB signaling pathway in HS rats
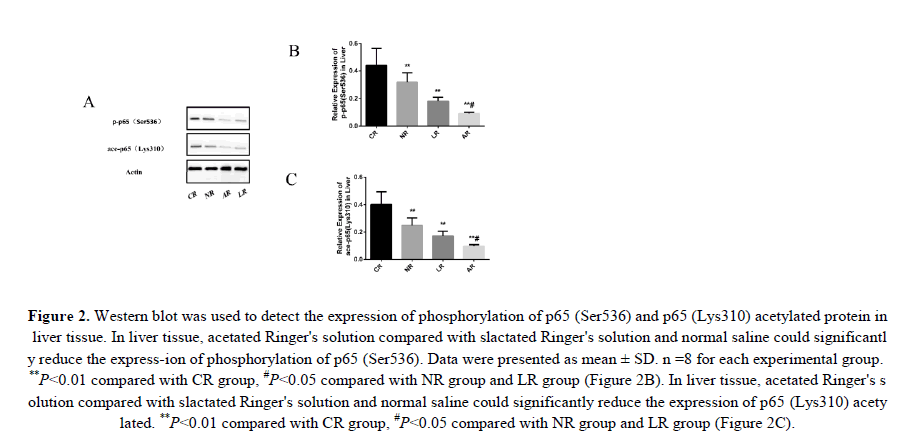
To test whether sodium acetated Ringer's solution could reduce the HS inflammatory response by inhibiting NF-κB, we assessed the protein expression levels of p65 (Ser536) phosphorylation and p65 (Lys310) acetylation in liver tissue. Phosphorylation and acetylation of p65 were important indicators of NF-κB signaling pathway activation. And phosphorylation of p65 (Ser536) enhanced the ability of NF-κB to regulate pro-inflammatory cytokines by promoting P65 (Lys310) acetylation. Obviously, HS induced activation of the NF-κB signaling pathway as shown f-rom change in these proteins. We could clearly see that acetated Ringer's solution compared to lactated ringer's solution and normal saline down-regulated p65 (Ser536) phosphorylation and p65 (Lys310) acetylated protein expression (Figure 2). Therefore, we demonstrated that sodium acetated Ringer's solution could inhibit the NF-κB signaling pathway.
Figure 2: Western blot was used to detect the expression of phosphorylation of p65 (Ser536) and p65 (Lys310) acetylated protein in liver tissue. In liver tissue, acetated Ringer's solution compared with slactated Ringer's solution and normal saline could significantly reduce the express-ion of phosphorylation of p65 (Ser536). Data were presented as mean ± SD. n =8 for each experimental group. **P<0.01 compared with CR group, #P<0.05 compared with NR group and LR group (Figure 2B). In liver tissue, acetated Ringer's solution compared with slactated Ringer's solution and normal saline could significantly reduce the expression of p65 (Lys310) acetylated. **P<0.01 compared with CR group, #P<0.05 compared with NR group and LR group (Figure 2C).
Discussion
The most common cause of severe traumatic early death is HS (Krug et al., 2000; Pfeifer et al., 2009). HS causes a sharp reduction in circulating blood volume, eventually leading to tissue damage and vital organ dysfunction (Douzinas et al., 2012). The liver is an important internal organ of the human body and is susceptible to HS. The liver is currently considered as one of t-he first organs subjected to hypoxia induced by hemorrhagic shock (Karmaniolou et al., 2013). Therefore, the study of HS-induced liver injury is of great significance for the further study of the SIRS and MODS caused by hemorrhagic shock.
Acetated Ringer's solution as a new balanced salt solution, compared with slactated Ringer's solution, to maintain a high levels of bicarbonate and reduce the amount of alkali deficiency, less lead to hyperglycemia (Kumar et al., 2016). And sodium acetate metabolism is seldom dependent on the liver (Kumar et al., 2016). Acetated Ringer's solution compared to lactated Ringer's solution can significantly reduce the body's inflammatory response (Cai et al., 2016). However, the mechanism of acetated Ringer's solution for restoring HS to reduce inflammatory injury h-as not been elucidated yet.
HS is characterized by early inflammatory response and some immune suppression (Zhang et al., 2014). NF-κB plays a major role in facilitating the transcription of many inflammatory factors (Bartuzi et al., 2013). NF-κB exists as an inactive complex of the p50 and p65 subunits and the inhibitory protein IκB-α. p65 has the strongest transcriptional activity and has strong DNA binding affinity (Sharma et al., 2015). In recent years, phosphorylation of NF-κB, especially p65 serine 536 phosphorylation has become a new mechanism of transcriptional activation (Sasaki et al., 2005). p65 binds to the transcriptional activator p300 and the CREB binding protein and can be modified by acetylation of certain lysine residues to produce different functional effects. p65 (Ser536) phosphorylation promotes p65 (Lys310) acetylation by increasing the binding of histone acetylase to p65 (Chen et al., 2005). Acetylation of p65 enhances the binding ability to DNA and further enhances the regulation of pro-inflammatory cytokines by NF-κB (Bhatt., 2014). In this study, acetated Ringer's solution and slactated Ringer's solution and normal saline for recovery of HS rats. Compared with normal saline and lactated Ringer's solution, resuscitation-n of acetated Ringer's solution could significantly reduce the phosphorylation of p65 (Ser536) and decrease the levels of p65 (Lys310) acetylation after 4 hours resuscitation. Our study shows that acetated Ringer's solution can down-regulate the phosphorylation levels of p65 (Ser536) in the liver of HS rats, thereby preventing the binding of histone acetylase to p65 and eventually reversing the acetylation of p65 (Lys310).
The release of NF-κB for inflammatory response and mediated inflammatory cytokines such as TNF-α, IL-1β and IL-6 is important and aggravates inflammatory lesions (Zhang et al., 2015; Belinga et al., 2016). TNF-α is secreted by activated macrophages and plays a pivotal role in a variety of biological effects and is considered a potent pro-inflammatory cytokine (Wu et al., 2015). Its expression reflects the severity of tissue damage and organ dysfunction, and liver injury leads to a significant increase in TNF-α (Liu et al., 2012). TNF-α can induce the degradation of NF-κB inhibitor (IκB), enhancing the activity of NF-κB, promoting the synthesis and release of TNF-α, IL-1 and IL-6, and ultimately forming cascade reaction (Schütze et al., 1995). In our previous study (Cai et al., 2016), it was shown that acetated Ringer's solution significantly reduced TNF-α levels compared to lactated Ringer's solution. This experiment further shows that lactated Ringer's solution compared with lactated Ringer's solution and normal saline can significantly inhibit NF-κB pathway through p65 (Lys310) deacetylation of NF-κB, resulting in the decrease of downstream inflammatory cytokines, and downregulated TNF-α mRNA levels.
Conclusion
IL-4 and IL-10 are important anti-inflammatory factors. IL-10 is a pleiotropic immunomodulatory cytokine that plays a key role in controlling inflammation and immune regulation (Couper et al., 2008). IL-4 and IL-10 can promote humoral immune response by down-regulating inflammatory mediators including TNF-α and IL-1, and can promote humoral immune response (Rogy et al., 2000). Our study shows that sodium acetate Ringer's solution recovery of HS rats, can significantly up-regulate IL-4, IL-10 mRNA levels and reduce TNF-α mRNA levels. Acetate Ringer's solution can reverse the imbalance of pro-inflammatory cytokines and anti-inflammatory cytokines, which can reduce the body's inflammatory response. However, this study did not combine the relationship between NF-κB and IL-4, IL-10 expression. In conclusion, acetate Ringer's solution may inhibit the activation of NF-κB, thereby inhibiting the NF-κB signaling pathway; inhibit the downstream-am inflammatory cytokine expression and synthesis, and upregulate of anti-inflammatory cytokines. Ultimately, acetate Ringer's solution attenuates the inflammatory reaction in the liver tissue of HS rats.
Conflicts of interest
Zhipeng Xu, Qi Song, Zhaolei Qiu, Lei Li, Zhaohui Du and Zhenjie Wang declare that they have no conflict of interest.
Acknowledgment
This work was supported in part by Anhui province science and technology key project (1604a0802089,Natural Science Foundation of Anhui province ( (No. KJ2015B092by).
ETHICAL STANDARDS
This study was approved by Bengbu Medical College Experimental Animal Management and Ethics Committee.
About the Authors
Corresponding Author
Z. J. Wang
The First Affiliated Hospital of Bengbu Medical College, Bengbu, Anhui, P.R. China
- Email:
- ahbyfywzj@163.com
References
- Cocchi MN, Kimlin E, Walsh M (2007). Identification and resuscitation of the trauma patient in shock. Emerg Med Clin North Am.25 (3): 623-42. https://doi. org/10.1016/j. emc.2007. 06. 001
- Angele MK and Schneider CP (2008). Bench-to-bedside review: Latest results in hemorrhagic shock. Crit Care. 12 (4): 218. https://doi. org/10.1186/cc6919
- Chen G, You G and Wang Y (2013) Effects of synthetic colloids on oxidative stress and inflammatory response in hemorrhagic shock: comparison of hydroxyethyl starch 130/0.4, hydr-oxyethyl starch 200/0.5, and succinylated gelatin. Crit Care. 17 (4): R141. https://doi. org/10.1186/cc12820
- Fukudome EY, Li Y and Kochanek AR (2012). Pharmacologic resuscitation decreases circulating cytokine-induced neutrophil chemo attractant-1 levels and attenuates haemorrhage-induced acute lung injury. Surgery. 152: 254-261. https://doi. org/10.1016/j. surg.2012. 03. 013
- El-Tahan RR, Ghoneim AM (2016). TNF-α gene polymorphisms and expression. Springerplus. 5: 1508. https://doi. org/10.1186/s40064-016-3197-y
- Liu FC, Chaudry IH (2017). Hepatoprotective effects of corilagin following hemorrhagic shock are through Akt-Dependent Pathway. Shock. 47: 346-351. https://doi. org/10.1097/shk. 0000000000000736
- Cai T, Wang ZJ and Wang L (2016). The effects of lactated Ringer's solution and acetated Ringer's solution resuscitation on TNF-α, IL-6 and IL-10 in hemorrhagic shock rats. Chin J Crit Care Med. 36: 166-170.
- Krug EG, Sharma GK (2000). The global burden of injuries. Am J Public Health. 90: 523-526. https://doi. org/10.2105/ajph. 90.4.523
- Pfeifer R, Tarkin IS, Rocos B (2009). Patterns of mortality and causes of death in polytrauma patients has anything changed? Injury. 40: 907-911. https://doi. org/10.1016/j. injury.2009. 05. 006
- Douzinas EE (2012). Hemorrhagic shock resuscitation: A critical issue on the development of posttraumatic multiple organ failure. Crit Care Med. 40: 1348-1349. https://doi. org/10.1097/ccm. 0b013e31823e9501
- Karmaniolou II, Theodoraki KA and Orfanos NF (2013). Resuscitation after hemorrhagic shock: the effect on the liver a review of experimental data. J Anesth.27: 447-460. https://doi. org/10.1007/s00540-012-1543-y
- Kumar L, Seetharaman M and Rajmohan N (2016). Metabolic profile in right lobe living donor hepatectomy: Comparison of lactated Ringer's solution and normal saline versus acetate based balanced salt solution a pilot study. Indian J Anaesth. 60: 719-725. https://doi. org/10.4103/0019-5049. 191669
- Zhang Y, Zhang J, Korff S, et al. (2014). Delayed neutralization of interleukin 6 reduces organ injury, selectively suppresses inflammatory mediator, and partially normalizes immune dysfunction following trauma and hemorrhagic shock. Shock. 42: 218-227. https://doi. org/10.1097/shk. 0000000000000211
- Bartuzi P, Hofker MH (2013). Tuning NF-κB activity: a touch of COMMD proteins. Biochim Biophys Acta. 1832: 2315-2321. https://doi. org/10.1016/j. bbadis.2013. 09. 014
- Sharma V, Jordan JJ and Ciribilli Y (2015). Quantitative analysis of NF-κB transactivation specificity using a yeast-based functional assay. PLoS One. 10: e0130170.https://doi. org/10.1371/journal. pone. 0130170
- Sasaki CY, Barberi TJ, Ghosh P (2005). Phosphorylation of RelA/p65 on serine 536 defines an I{kap-pa}B{alpha}-independent NF-{kappa}B pathway. J Biol Chem. 280: 34538-34547. https://doi. org/10.1074/jbc. m504943200
- Chen LF, Williams SA and Mu Y (2005). NF-kappaB RelA phosphorylation regulates RelA acetylation. Mol Cell Biol.25: 7966-7975.
- Bhatt D (2014). Regulation of the NF-κB-mediated transcription of inflammatory genes. Front Immunol. 5: 71. https://doi. org/10.3389/fimmu.2014. 00071
- Zhang YF, Zou XL and Wu J (2015). Rosiglitazone, a peroxisome proliferator-activated receptor (PPAR)-γ Agonist, Attenuates Inflammation Via NF-κB Inhibition in Lipopolysaccharide Induced Peritonitis. Inflammation.38: 2105-2115. https://doi. org/10.1007/s10753-015-0193-2
- Belinga VF, Wu GJ, Yan FL (2016). Splenectomy following MCAO inhibits the TLR4-NF-κB signaling pathway and protects the brain from neurodegeneration in rats. J Neuroimmunol.293: 105-113. https://doi. org/10.1016/j. jneuroim.2016. 03. 003
- Wu X, Xu W, Feng X, et al. (2015). TNF-a mediated inflammatory macrophage polarization contributes to the pathogenesis of steroid-induced osteonecrosis in mice. Int J Immunopathol Ph-armacol.28: 351-361. https://doi. org/10.1177/0394632015593228
- Liu FC, Yu HP, Hwang TL (2012). Protective effect of tropisetron on rodent hepatic injury after trauma-hemorrhagic shock through P38 MAPK-dependent hemeoxygenase-1 expression. PLoS One. 7: e53203. https://doi. org/10.1371/journal. pone. 0053203
- Schütze S, Wiegmann K and Machleidt T (1995). TNF-induced activation of NF-kappaB. Immun-obiology. 193: 193-203.
- Couper KN, Blount DG (2008). IL-10: The master regulator of immunity to infection. J Immunol. 180: 5771-5777. https://doi. org/10.4049/jimmunol. 180.9. 5771
- Rogy MA, Beinhauer BG and Reinisch W (2000). Transfer of interleukin-4 and interleukin-10 in patients with severe inflammatory bowel disease of the rectum. Hum Gene Ther. 11: 1731-1741. https://doi. org/10.1089/10430340050111386
Keywords:
Download:
Full PDF- Share This